The high-throughput and increasingly affordable nature of next-generation sequencing (NGS) has led to its expanded use in routine clinical procedures.
The relative simplicity of targeted enrichment cancer panels (available from a number of commercial providers) allows routine laboratories to simultaneously analyze the coding (exonic) regions of multiple cancer-related key genes. Combine this with the statistic diagnostic testing now influencing over 70 percent of all health care decisions1, the setting up or transitioning to NGS-based oncology panels for labs has never been more important.
Due to the complex mechanisms driving carcinogenesis, multiple aberrations in tumor suppressor genes are increasingly becoming important and are relevant for tumor diagnostics and therapy. Therefore, to enhance the understanding of the underlying pathogenesis, extensive investigation of commonly mutated and critical key genes are an efficient way to analyze the oncogenic potential in human cancer samples.
Sanger sequencing vs. oncology panels
So why are people transitioning from Sanger sequencing to NGS/panel-based sequencing?
Aside from the points already mentioned, at present, the total number of genes relevant to targeted therapies, diagnostic and prognostic implications and clinical trials is in the ~hundreds. Although to note, for any given cancer type, this number may be far lower: in some cases only half a dozen or so. As such, the majority of variants or mutations detected by genome-wide sequencing of neoplastic specimens cannot be interpreted in a clinical setting and are of limited clinical utility. Therefore labs are instead looking at oncology panels for their answers of cost-effective sequencing of an increasing number of variants/genes.
To note: For rare cancer types or types with unusually broad mutational spectra, genome-wide sequencing may already have clinical use enough above panels to merit semi-routine implementation.
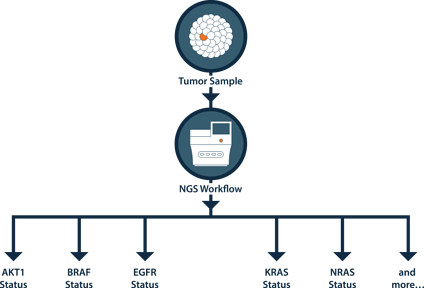
NGS Advantage
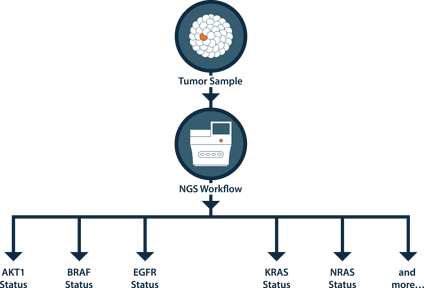
Figure - Using just one sample, one workflow can test for mutation status across multiple genes.
NGS Advantage
NGS can call other mutations in EGFR, KRAS and BRAF that represent standard-of-care that can go undetected by Sanger/qPCR methods.
Comparing the advantages and disadvantages of oncology panels
A simplistic short table summarizing the advantages/disadvantages of NGS compared to Sanger sequencing can be made to explain why many labs are transitioning over to NGS, but also the complexity of doing so.
|
|
Sanger sequencing | NGS |
|---|---|---|
|
Maximum read length |
Maximum ~1kb |
Massively parallel (~300Gb of DNA) |
|
Input amount |
Hundreds of copies of gene |
Single strand of template DNA |
|
Speed |
Slow |
Fast (whole genome sequencing) |
|
Cost per run |
Low |
High |
|
Cost per sample/bp |
High |
Low (if multiplexed) |
|
Preparation steps |
Few |
Many (complicated) |
|
Complexity |
Low |
High |
|
Initial set-up |
Low |
High |
Table - the advantages and disadvantages of NGS-based oncology panels
Although the advantages of NGS-based oncology panels are evident and commonly accepted, the setting-up and validation of a panel can bring new challenges, expertise needs and problems. Some of the most common challenges of an NGS workflow are summarized below, with a summary of each stage.
Challenges of an NGS workflow
| |
Tumor sample Heterogeneity (stromal contamination), low quantity and poor sample quality are key factors that impact the final results of the assay. |
| |
DNA extraction Extraction from low-quality and low-quantity samples, along with accurate assessment of quantity are challenges for patient-derived samples. |
| |
Library preparation The specific library preparation approach is tailored both to the goals of the experiment and the sample quantity/quality available. In each case, platform-specific adapters must be incorporated. |
| |
Sequencing Read length and type (paired-end vs single-end), along with sample multiplexing is determined by the library fragment size and the appropriate coverage required for detection. |
| |
Bioinformatics On-machine informatics analysis may be employed, or the data may be exported and fed into commercially available software or a home-brewed pipeline. |
| |
Analysis and interpretation After generating a list of variant calls and their corresponding frequencies, database annotations, statistics and metadata may be incorporated to better understand and interpret the results of the assay. |
What is available to meet the evolving needs of NGS?
In order to help labs, multiple options exist to meet the new and evolving validation and optimization needs of oncology panels, including: cell lines, reference standards, patient samples and oligonucleotides each with their own advantages and disadvantages. Furthermore, dependent on each laboratories' accreditation or requirements they recommend different techniques. For example, The New York State guidelines state:
"Prior to final validation non-patient defined mixtures of cell line DNAs (not plasmids) can be used to will help with initial optimization and understanding of precision for the detection of variants"
A comparison of oncology panel's performance during validation
During validation studies and due to its complexity, NGS requires several steps including those that demonstrate the effective and reproducible performance of the NGS workflow.
To demonstrate the importance of monitoring a panel's performance, at Horizon, we compared two different panels (Ampliseq Cancer Panel, Ampliseq Cancer Hotspot Panel v2 (x2)) across three labs using a single source of reference material: the FFPE Quantitative Multiplex reference standard and reported results.
| Platform: |
|
QX100 Droplet Digital PCR |
Ampliseq Cancer Panel |
Ampliseq Cancer Hotspot Panel v2 |
Ampliseq Cancer Panel v2 (Average of 8 runs) |
|
|---|---|---|---|---|---|---|
| Sequencing Depth: |
|
N/A |
3000-4000x |
Average 5000x |
2000x |
|
| Gene | Mutation | Specification | Observed mutant ratio | |||
|
BRAF |
V600E |
10.5 |
10.2 |
9.9 |
9.1 |
10.3 |
|
KIT |
D816V |
10.0 |
10.4 |
10.0 |
11.0 |
10.1 |
|
EGFR |
ΔE746 - A750 |
2.0 |
2.0 |
2.3 |
Not detected |
Not detected |
|
EGFR |
L858R |
3.0 |
2.7 |
2.7 |
2.1 |
2.4 |
|
EGFR |
T790M |
1.0 |
0.9 |
0.8 |
Not detected |
Not detected |
|
EGFR |
G719S |
24.5 |
24.4 |
23.7 |
23.1 |
24.8 |
|
KRAS |
G13D |
15.0 |
16.1 |
16.3 |
12.35 |
15.5 |
|
KRAS |
G12D |
6.0 |
5.0 |
5.2 |
Not detected |
5.1 |
|
NRAS |
Q61K |
12.5 |
12.8 |
9.0 |
12.7 |
12.6 |
|
PIK3CA |
H1047R |
17.5 |
18.6 |
16.7 |
16.8 |
17.9 |
|
PIK3CA |
E545K |
9.0 |
8.9 |
3.2 |
8.4 |
8.8 |
Table - Quantitative Multiplex Reference Standard sequenced across 2 panels in 3 labs
In line with additional validation steps, the clinical laboratory has successfully implemented the NGS analysis workflow to simultaneously analyze the mutational status of multiple cancer related key genes and intends to use FFPE reference standards to routinely monitor NGS assay performance.
Implementing an oncology panel in your laboratory
If you're just getting started and are starting to implement an oncology panel(s) in your laboratory, find out how one lab is using reference material to implement clinical oncology panels.
