Are you facing the problem of limited resources or expertise to tackle complex immune cell projects? Partner with our team of experts to reach the frontier in your field and overcome your challenges. Consult with our dedicated project team to define project scope, timelines, and goals. Together, we'll craft a roadmap for success and navigate the journey toward exceptional outcomes.
Receive frequent and regular status updates, fostering transparent and effective communication. Stay informed about the project's progress, milestones, and significant developments, enabling you to make informed decisions and provide timely feedback.
Obtain a comprehensive and in-depth analysis of results, delivered with our commitment to reliable data. Our rigorous analytical methods ensure accurate and meaningful interpretations, empowering you to make data-driven decisions and drive future strategies.
Get the benefits of outsourcing with us
- Increased efficiency: Focus on your competencies on what you do best while we handle specialized tasks.
- Specialized access: Tap into our global talent pool for diverse skills and knowledge.
- Scalability and flexibility: Adjust your operations based on business needs with ease.
- Risk sharing: Transfer certain risks to our capable hands, minimizing your burden.
- Time savings: Free up your time for strategic activities while we take care of time-consuming tasks.
Our immune cell custom screening advantages
- Dedicated project management team
- Miniaturized primary immune cell assays platform
- Donor specifications
- Imaging readouts
- Leverage the reliability of ImmuSignature™ assays
- Extensive library of 600+ cell lines
Examples of assay development areas
Consult with our scientist to develop your next screening assay in the following areas of primary immune cells.
- T cell-mediated tumor-killing assays
- B cell polarization and suppression assays
- M1 and M2 macrophages polarization
- NK cell Antibody-Dependent and Cell-mediated Cytotoxicity (ADCC) assays
- Antibody-dependent cellular phagocytosis (ADCP)
- 3D T cell infiltration assay.
Explore our capabilities
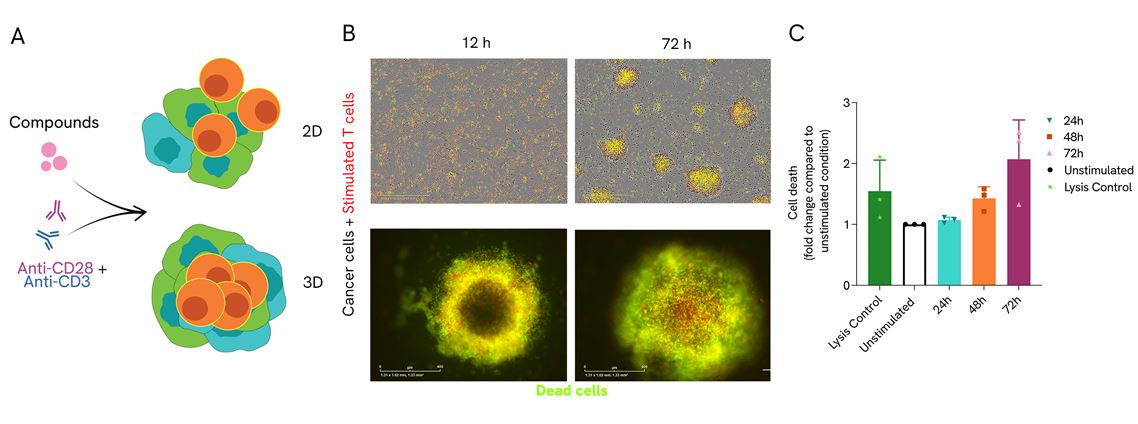
T cells killing assay in 2D and 3D configuration. A) Human CD3+ T cells were co-cultured in a monolayer of OncoSignatureTM cancer cell lines (2D) or OncoSignatureTM cancer cell spheroids (3D) and stimulated with anti-CD3/CD28. B) Representative IncuCyte S3 images show T cell-mediated cancer cell killing in stimulated T cell condition. CD3+ T cells are depicted in red, dead cells in green. White arrows indicate CD3+ T cell infiltration into the spheroids. C) Kinetic cell death was quantified using CellToxTM Green dye on Omega FLUOstar®. Data is depicted from three human T cell donors.
An arrayed B cell CRISPR screening service to help you understand the critical roles of B cells, including regulatory B cells (Bregs), in modulating immune responses, tumor responses and/or in auto-immune disorders. Identify genes that, when knocked out, affect the B cell function and gene in B cells that affect the function of another immune cell.
Providing a service that includes:
- Gene editing of human stimulated B cells
- In an arrayed format with synthetic crRNA-tracrRNA guides
- Standard screen includes 50 genes per donor or customized functional screens
- Option to co-culture the edited cells with other immune cells
- Data delivery between 14-18 weeks
The Complement-dependent cytotoxicity (CDC) assay is a powerful screening platform to aid the development of successful therapeutic mAbs. Here, we have developed a robust CDC assay workflow incorporating semi-automation, which accurately and reproducibly supports the testing and comparison of mAbs candidates across a panel of cancer cell lines. The CDC assay facilitates the validation of therapeutic antibodies for cancer immunotherapy, confirming complement-dependent effectiveness and identifying the mechanism of action in model cancer cell lines. Additionally, screening therapeutic candidates in a broader OncoSignature™ panel of cancer cell lines expands the applications of your lead compound to other cell types or tissues.
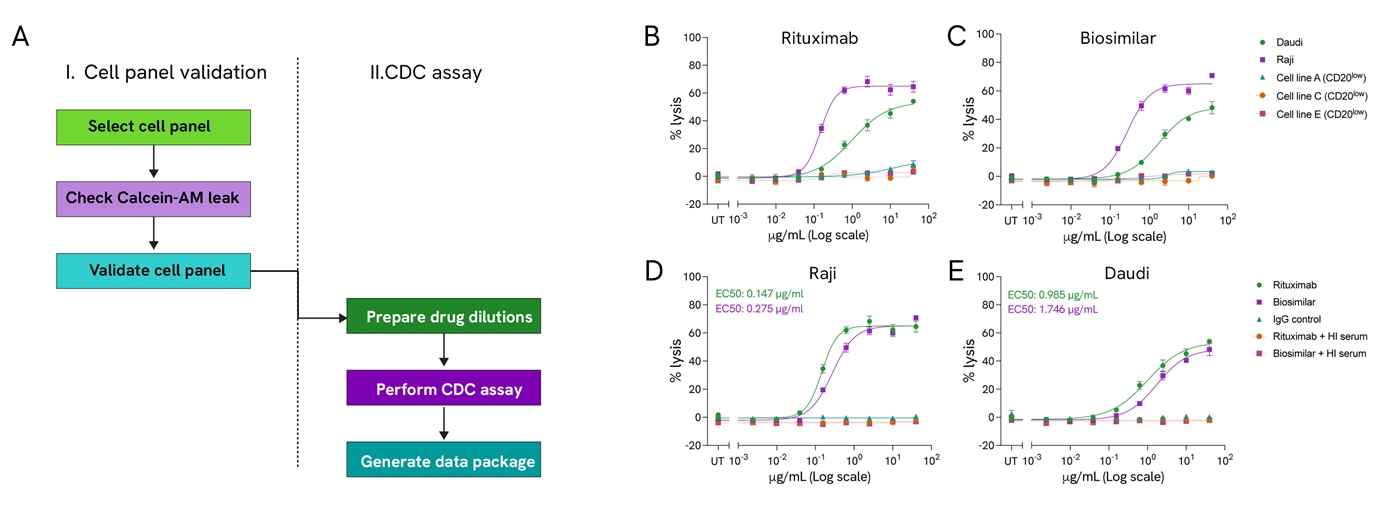
Complement-dependent cytotoxicity (CDC) assay: A) The CDC assay workflow has two phases: validating the cell lines' ability to release calcein and a second stage where testing compounds challenge the selected cell lines. Rituximab (B) and its biosimilar (C) show similar complement-dependent lysis percentages in five cell lines. Percentage lysis in Raji (D) and Daudi cells (E) treated with rituximab, biosimilar, IgG control, rituximab and HI serum, biosimilar and HI serum plotted over drug concentration. EC50 values for rituximab (green) and biosimilar (purple). UT: Untreated.
Learn more