- CRISPRa (CRISPR activation) 試薬
- CRISPRmod CRISPRa Synthetic sgRNA non-targeting Controls
CRISPRmod CRISPRa Synthetic sgRNA non-targeting Controls
Non-targeting controls to evaluate baseline cellular responses to CRISPRa components in the absence of gene target-specific sgRNA
CRISPRa synthetic sgRNA non-targeting controls are recommended as negative controls for experiments using CRISPRa synthetic sgRNAs in human and mouse cells. They are designed to have a minimum of three mismatches or gaps to all potential PAM-adjacent targets in human, mouse and rat genomes. When using these controls, changes in cellular viability or gene expression likely reflect nonspecific cellular responses that can be used as a baseline for comparison to cells treated with target-specific reagents.
Highlights
- Proprietary alignment tools used to verify at least three mismatches or gaps to any potential target in human, mouse or rat genomes
- Choose an individual or pooled sgRNA control to match your experimental sgRNA reagent
CRISPRa workflow diagram with stable dCas9-VPR expression
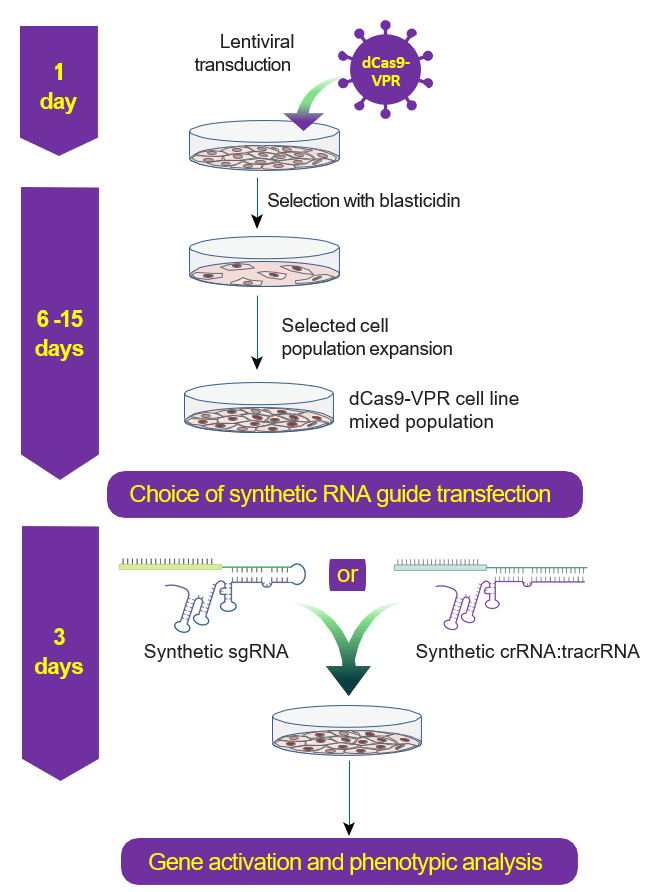
CRISPR activation workflow with Lentiviral dCas9-VPR and synthetic crRNA:tracrRNA (right side) or Lentiviral expressed sgRNA (left side).
CRISPRa plasmid co-transfection workflow
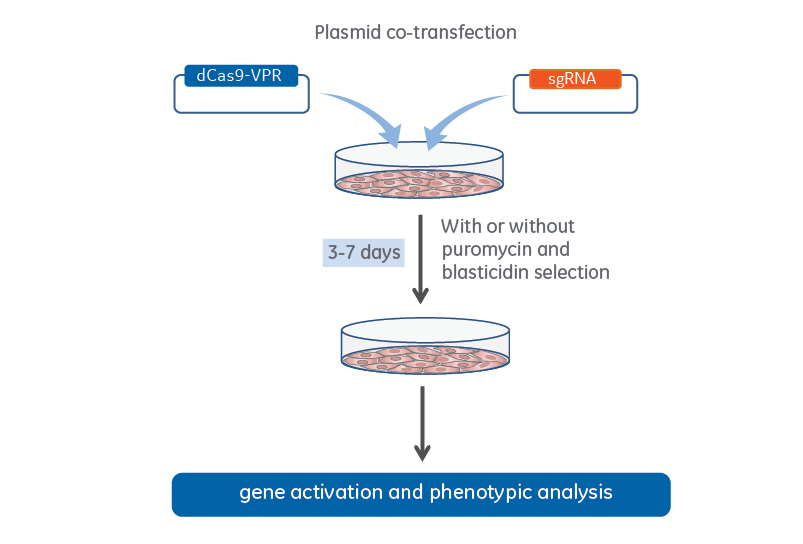
CRISPRa workflow for plasmid co-transfection of lentiviral dCas9-VPR and expressed sgRNA.
How much CRISPRa sgRNA do I need?
This table provides the approximate number of experiments that can be carried out for lipid transfection methods at the recommended sgRNA working concentration (25 nM) in various plate/well formats using either a pool or individual sgRNA. Calculations do not account for pipetting errors.
| sgRNA nmol | 96-well plate 100 µL volume | 24-well plate 500 µL volume | 12-well plate 1000 µL volume | 6-well plate 2500 µL volume |
|---|---|---|---|---|
| 2 | 800 | 160 | 80 | 32 |
| 5 | 2000 | 400 | 200 | 80 |
| 10 | 4000 | 800 | 400 | 160 |
Individual CRISPRa crRNAs achieve robust target gene activation on their own, or when pooled together in a single reagent

NIH-3T3 cells stably expressing integrated CRISPRa dCas9-VPR (driven by mCMV promoter) were plated at 10,000 cells/well and transfected using DharmaFECT 1 Transfection Reagent (0.2 uL/well) with CRISPRa synthetic single guide RNA (sgRNA) targeting ttn or pou5f1 genes. Four CRISPRa sgRNAs were used either individually or pooled (to an individual or pooled concentration of 25 nM). Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
Gene activation using CRISPRa synthetic single guide RNA in dCas9-VPR stable human and mouse cell lines

Human U2OS or mouse NIH-3T3 cells stably expressing integrated CRISPRa dCas9-VPR (driven by hEF1a promoter in human and mCMV promoter in mouse cells) were plated at 10,000 cells/well and transfected using DharmaFECT 1 Transfection Reagent (0.2 uL/well) with 25 nM individual or pooled CRISPRa synthetic single guide RNA (sgRNA) targeting either human (TTN or POU5F1) mouse (pou5f1 or ttn) gene targets. Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
sgRNA dose response curve demonstrates effective gene activation at 25 mM sgRNA concentration
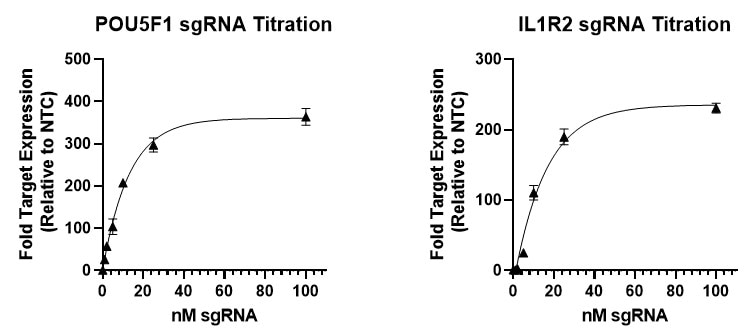
U2OS-dCas9-VPR stable cells were plated at 10,000 cells/well and transfected using 0.2 uL/well DharmaFECT 1 Transfection Reagent with CRISPRa synthetic single guide RNA (sgRNA) targeting POU5F1 or IL1R2 at six concentrations (0, 1, 5, 10, 25 and 100 nM). At 72 hours post-transfection, cell lysates were used to generate total cDNA, from which target gene expression was assessed using RT-qPCR. Relative expression of each gene was calculated with the Cq method using beta-actin mRNA as the housekeeping gene and normalized to a non-targeting control. Data points were fit to a one phase exponential decay equation.
Robust CRISPRa gene activation peaks between 24 to 72 hours
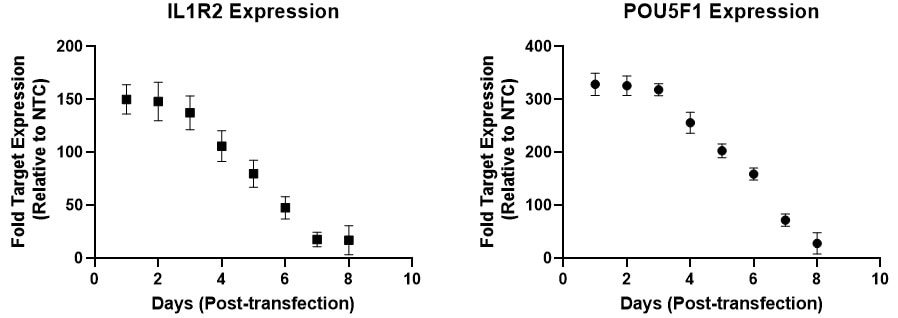
U2OS-dCas9-VPR stable cells were plated at 5,000 cells/well and transfected using 0.2 uL / well DharmaFECT 1 Transfection Reagent with 25 nM CRISPRa synthetic single guide RNA (sgRNA) targeting POU5F1, IL1R2, or non-targeting control (NTC). Transfected cells were passaged every 2-3 days to avoid overconfluency. Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
