Mimix NIPT標準サンプル
患者類似の非侵襲的出生前検査(NIPT)用標準サンプル
Revvityは、Mimix™ NIPT標準サンプルを開発しました。これは、母体血から胎児の染色体異常(トリソミーなど)やその他の染色体異常を検出する分子研究ワークフローの検証・管理を支援する、母体・胎児由来の標準サンプルです。
トリソミー13、トリソミー18、トリソミー21といった、出生前検査で一般的に対象となる染色体異常をカバーしており、幅広い出生前アッセイ手法の検証、モニタリング、トラブルシューティングに活用できます。
NIPT標準サンプルの重要性
NIPT(非侵襲的出生前検査)は、母体血漿中の胎児由来DNAを用いて胎児の染色体異常をスクリーニングする方法であり、侵襲的な羊水検査や絨毛検査(CVS)と比較して、流産のリスクを伴わないスクリーニング手法です。
母体血中では、母体由来のcell-free DNA (cfDNA)が多く存在する中で、微量の胎児由来cfDNAから異常を検出する必要があり、これは大きな技術的課題です。そのため、NIPTの結果が一貫性と精度を持っていることを体系的に保証する必要があります。
Revvityの標準サンプルは、トリソミー13(Patau症候群)、トリソミー18(Edwards症候群)、トリソミー21(Down症候群)、および正常核型(ユープロイド)の検出において、スクリーニング検査が期待通りに機能しているかを確認するために役立ちます。
Mimix NIPT 標準サンプルの用途
- 出生前アッセイの分析感度と精度の評価
- 遺伝学的出生前研究アッセイの検証・モニタリング・トラブルシューティング
- 母体・胎児サンプルを模倣した胎児DNA比率の設定によるアッセイのベースライン構築
- 研究ワークフロー全体の支援
RevvityのNIPT標準サンプルは、ddPCRやVanadis™ NIPTシステム**を用いた広範な検証により、以下の目的に活用されています:
- 研究対象の個別選定の支援
- 治療効果の評価
- ワークフロー全体の検証管理
- 母体・胎児由来cfDNAをベースとした標準サンプルによる研究ワークフローの信頼性確認
- データの誤解釈リスクを低減する、比較可能かつ再現性のある結果の提供
Mimix 標準サンプルが選ばれる理由
- 母体血サンプルに近い構成を持つ、細胞株由来のコントロール
- 合成血漿中に含まれる、母体・胎児由来cfDNAの持続可能な供給源
- 40 ng/mLの濃度、170 bpのサイズ分布、10%の胎児DNA比率で再現性のあるコントロールを提供
- ISO 9001:2015準拠の品質管理体制で製造
Vanadis™ NIPTシステムによる検出結果
Vanadis™ NIPTシステム**は、PCR、シーケンシング、マイクロアレイ、マイクロ流体技術を使用せずに、cfDNAのターゲット解析を可能にし、染色体21、18、13の定量を実現します。このシステムでは、各染色体に対して約3,500個のcfDNA断片を高い特異性を持つ蛍光標識で検出します。専用のナノフィルタープレートが標識されたDNA分子を捕捉し、イメージングによって密度スコアを算出します。

*製品ごとの平均正規化比率
Vanadis™ NIPTシステム**を用いて、RevvityのMimix NIPT標準サンプルで得られた代表的なデータを示します。正規化比率は、Vanadis NIPTシステム**のイメージングデータと染色体13、18、21の密度スコアをもとに算出され、各染色体のZスコアの判定に使用されています。
 |
 |
 |
Vanadis™ NIPTシステム**とMimix NIPT標準サンプルで得られたデータを用いた代表的な正規化比率プロット。解析対象の染色体(13、18、21)およびユープロイドのデータポイントは、対角線上またはその近傍に位置することが望ましく、ユープロイドはプロットの中心付近に、染色体異常が陽性の場合は、解析対象の染色体が右上の象限に位置します。
 |
 |
 |
 |
Vanadis™ NIPTシステム**とMimix NIPT標準サンプルで得られたデータを用いた代表的なZスコアプロット。トリソミー13、18、21が陽性となるすべてのサンプルは、Zスコアが3.15または3.5を超えています。
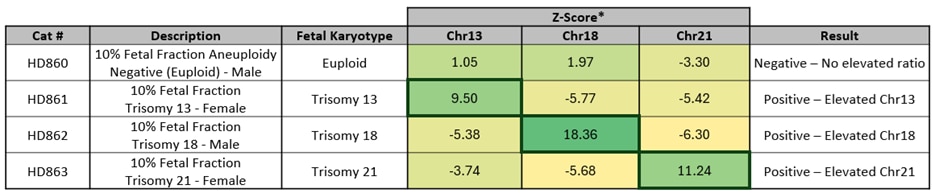
*製品ごとの平均Zスコア
**Vanadis製品は、米国やカナダなど、すべての国において法律に準拠した認可を受けているわけではありません。 詳細は、お近くの代理店にご確認ください。
NIPT製品のご注文
これらのcfDNA標準サンプルは、最も一般的な3種類の染色体異常を模倣しています。各製品は、母体・胎児由来の特定の細胞株セットに基づいています。
正常染色体男性由来標準サンプル
このコントロールはトリソミー13、18、21の染色体異常が陰性であり、健康なドナー由来の母体・胎児cfDNAを提供します。
Helpful resources
Vanadis NIPTシステム
Vanadis NIPTシステムは、染色体の定量を目的としたセルフリーDNA解析ソリューションであり、使いやすくスケーラブルなプラットフォームとして、研究施設に貢献します。
高精度NIPTのための単一DNA分子のイメージング
Dahl, F. et al. Imaging single DNA molecules for high precision NIPT. Sci Rep 8, 4549 (2018).
