Design rules improve the efficiency, specificity and functionality of gene knockout
Although CRISPR-Cas9 has become widely used for targeting specific genes for loss-of-function studies, the effectiveness of guide RNA reagents at creating double-strand breaks (DSBs) that result in a functional protein knockout depend heavily on the design of the guide RNAs. Using guide RNAs that have been designed based on an algorithm trained to score and select those RNAs that will give functional, specific knockout can make the difference between a conclusive experiment or a confounding one.
We have compiled a list of the top 5 reasons to use algorithm-designed guide RNAs, like the Dharmacon™ Edit-R™ product lines, to ensure the best chance of a successful knockout experiment.
-
Increased efficiency of creating DSBs
If you only use the “early exon” rule to design a guide RNA, how many more cells will you have to sort through and characterize in order to find one with the edit you need? Higher efficiency of editing means less work downstream; less work means faster results with lower total expenditure.The data below demonstrate that the guide RNAs that have high functionality scores from our Edit-R algorithm have correspondingly higher editing efficiency (shown by NGS analysis) than lower scoring designs.
-
Higher likelihood of functional gene knockout
Not all DSBs result in imperfect repair that leads to functional gene knockout. The data below demonstrates that although the indicated early-exon guide RNAs gave good indel formation (A), a secondary start site enabled the gene to produce a functional protein as demonstrated by the lack of GFP signal when these guide RNAs were used in a GFP reporter assay (B). GFP signal would indicate disruption of the functional protein, and these guide RNAs (indicated by red circle) did not induce a phenotype. -
Better specificity checking = avoidance of off-targets
Not all online gRNA design tools incorporate equivalent alignment methods to identify designs that will only target the site of interest. While nearly all online tools will identify potential off-targets with a perfect match or 1-3 mismatches, the Edit-R alignment tool is the only one that will also identify off-targets due to gaps or bulges between the guide RNA and the DNA target. If the tool cannot predict an off-target (and avoid it) how can it be specific and the user confident in the results? The Edit-R algorithm reduces the chance of off-targets with more specific designs from more thorough alignment analysis.
-
No design step required = ease of use and flexibility of format, scale and scope for your experiment
Instead of having to use a third party tool to design your guide RNAs, potentially using some guesswork as to which ones you should choose, with Edit-R predesigned guide RNAs you simply search for your gene and add the products to your cart. No extra steps, no custom orders, just access to highly efficient, specific, and functional crRNAs or sgRNAs.Since the Edit-R algorithm allows for the predesign of crRNAs and sgRNAs across the genomes of human, mouse and rat gene, we are able to offer a wide range of formats and scales for your gene editing experiment. Simply search for your gene of interest to get started! Working with more than a few genes? No problem. Browse our predefined mouse and human libraries or assemble a customized cherry-pick collection with your own gene list.
-
Get great gene knockout, regardless of the guide RNA format
The rules developed to select highly functional guide RNAs can be applied to both synthetic crRNAs and expressed sgRNAs, so regardless of which product format is best suited to your experiment and your cell type, you can be confident that the Edit-R predesigned guide RNA of choice will have all of the advantages of the Edit-R algorithm.

crRNA with high scores from the Edit-R algorithm have higher cleavage efficiency than low-scoring design
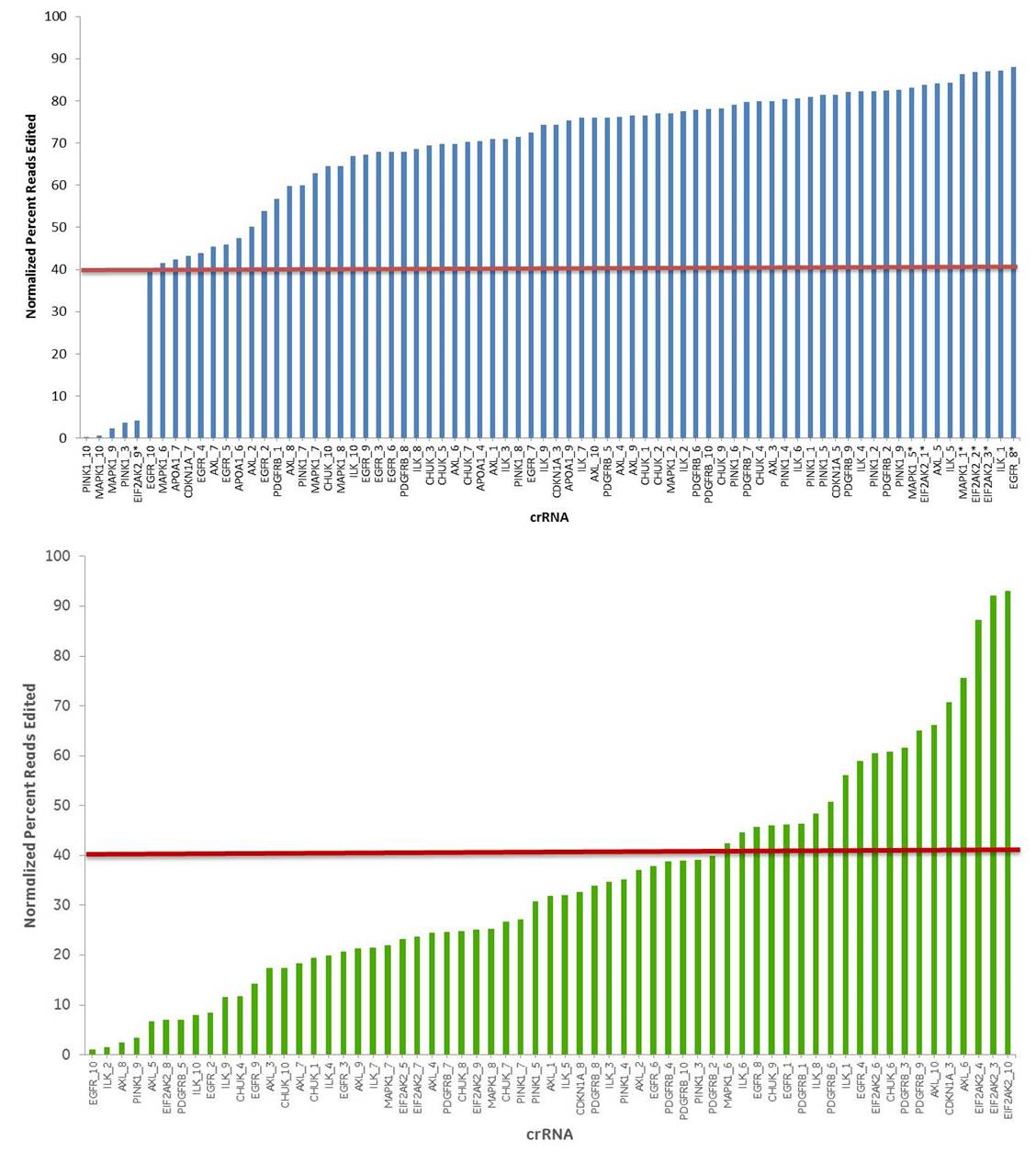
crRNA with high scores from the Edit-R algorithm have higher cleavage efficiency than low-scoring design

Creation of indels does not always result in functional gene knockout
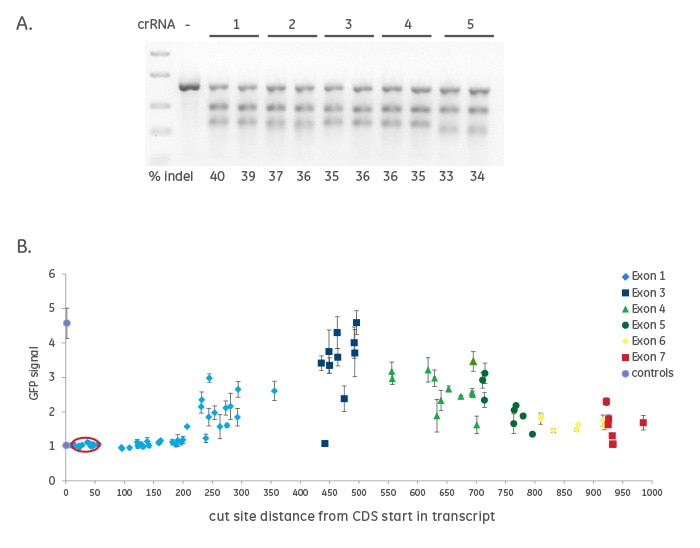
Figure 1. A recombinant U2OS reporter cell line (U2OS Ubi[G76V]-EGFP) that stably expresses Cas9 nuclease was used to determine cutting efficiency and functional protein knockout using crRNAs. This cell line also expresses a mutant ubiquitin fused to EGFP, such that when the proteasome pathway is functional, ubiquitin is degraded and there is no GFP signal. When the proteasome function is disrupted, there is accumulation of GFP and a measurable signal. crRNAs (88) were designed to every NGG PAM sequence spanning the PSMD8 gene, known to be necessary for proteasome function. These crRNAs, along with synthetic tracrRNA (final concentration 50 nM), were transfected into U2OS Ubi[G76V]-EGFP-Cas9 cells and analyzed 3 days post-transfection for gene editing and/or functional gene knockout. A. Five different synthetic crRNAs near the ATG start site in the first exon of PSMD8 [red circle in (B)] were harvested and assessed for CRISPR-Cas9-mediated indel formation with a mismatch detection assay using T7 Endonuclease I. Biological duplicates of each crRNAs were run on a 2% agarose gel and the gene editing efficiency was estimated to ~ 35% indicating that all five crRNAs result in gene editing. B. GFP intensity was measured for all 88 crRNAs. The higher the GFP signal indicates greater protein knockout and higher functionality of the crRNA. Significant variability in GFP intensity was observed for different crRNAs, demonstrating that not all crRNAs result in functional gene knockout, even if there is high efficiency of indel formation (A). These data indicated that an algorithm could be developed and used to choose functional crRNAs, not just for cutting.
Creation of indels does not always result in functional gene knockout

Gaps cause off target cleavage
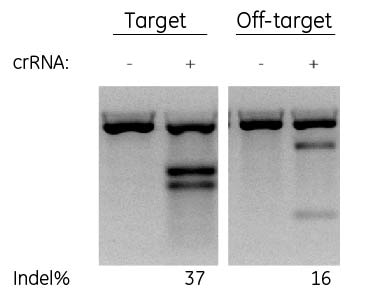
Gaps cause off target cleavage

Algorithm applies to both synthetic crRNAs and expressed sgRNAs
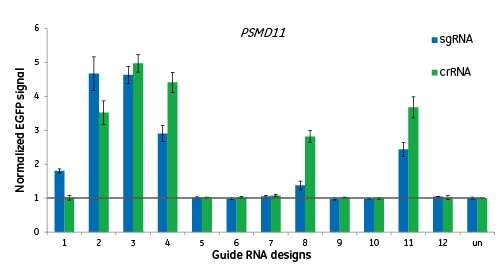
Here we are targeting the gene PSMD11 at 12 different sites and measuring functionality by EGFP signal to indicate disruption of the proteasome. Cells were either transfected with crRNA:tracrRNAs, or transduced with lentiviral particles for expression of a sgRNA of the same design. There is no significant difference between the two guide RNA formats when targeting the same genomic site; a good design for crRNA will translate into a good design for sgRNA.
Algorithm applies to both synthetic crRNAs and expressed sgRNAs
Additional Resources
- CRISPR-Cas9 gene editing products
Optimized tools for high-confidence genome engineering - An algorithm for selecting functional and specific CRISPR-Cas9 guide RNAs - Poster
Review our poster on selection of optimal CRISPR-Cas9 reagents for improving experimental outcomes - Improve CRISPR-Cas9 experiments with rationally designed guide RNAs - Webinar
CRISPR-Cas9 gene editing is a powerful technique that enables gene knockouts with greater speed and accuracy than previous approaches. Dharmacon has developed key technologies to improve CRISPR-Cas9 mediated gene knockout.
