Isolation of genomic DNA from formalin fixed paraffin embedded (FFPE) tissues is a critical step for molecular diagnostic (MDx) assays. It is essential that the maximum amount of DNA is recovered from the FFPE tissues and its quality is sufficient to perform the necessary MDx assays (e.g. QPCR, NGS, Sanger Sequencing, Pyrosequencing).
To investigate which DNA extraction kit was most appropriate for recovering the optimum DNA yield and concentration from low cell numbers, Horizon generated a custom FFPE cell line block, and performed extraction of DNA from FFPE using five comparable methods:
- Promega Maxwell16 FFPE Tissue LEV DNA Purification Kit
- Promega MagneSil Genomic, Fixed Tissue System Kit
- Promega ReliaPrep FFPE gDNA Miniprep System
- Qiagen DNeasy Blood & Tissue Kit
- Roche Cobas Sample Preparation Kit
Generation of a homogenous FFPE cell line material
KRAS (G12D/+) SW48 cells were grown, formalin fixed and embedded in paraffin. 10 μm sections from the FFPE block were cut, each containing approximately 100,000 cells. Six sections were analysed using each of the DNA extraction kits.
All extractions were performed according to the manufacturer's instructions. DNA recovery was measured using the Promega QuantiFlour DS Assay according to the manufacturer's instructions. (Note: Horizon's current product offering has been increased to 15 μm sections)
Analysis of DNA yield using different FFPE extraction kits
Figure 1 shows the average DNA recovered from six FFPE section using the Maxwell, Magnesil, Reliaprep, DNeasy or Cobas kits. The automated Promega Maxwell platform recovered the highest quantity of DNA.
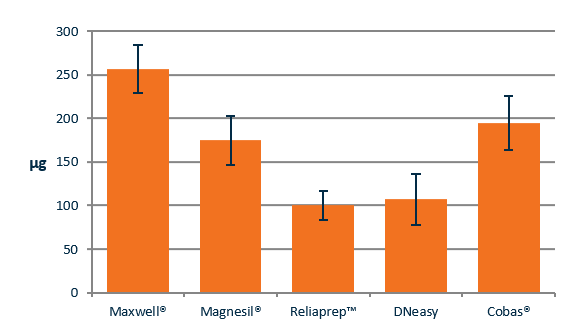
Comparison of DNA concentration using different FFPE extraction kits
Figure 2 shows the average DNA concentration recovered from six FFPE section using the Maxwell, Magnesil, Reliaprep, DNeasy or Cobas kits. The manual Promega Magnesil platform recovered the most concentrated DNA eluate.
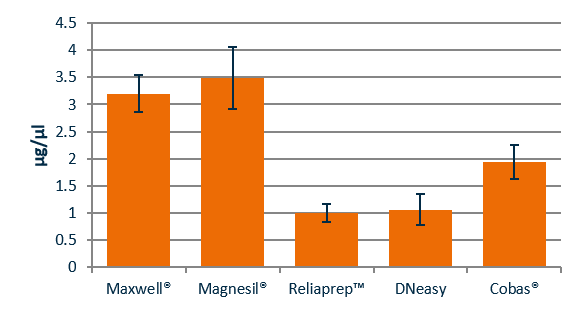
Comparison of bench time for different FFPE extraction kits
Although the protocol time (2-3 hours) for processing six samples is very similar between all five kits (excluding DNeasy which requires an overnight proteinase K step) the automated Maxwell platform out-performs the manual methods for larger sample numbers. Figure 3 shows the protocol time and the hands on time required to process thirty-two FFPE samples using each of the five kits. The protocol time and hands on time of the Maxwell platform is significantly shorter than the manual methods.
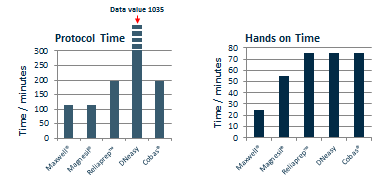 Conclusion
Conclusion
We believe Horizon's reference standards provided the ideal material with which to perform these kinds of comparative studies. The manufacturing process of our FFPE reference standards ensures that every section contains identical cell numbers and hence recoverable DNA quantity.
Using a low cell custom FFPE block we have been able to identify the Maxwell 16 FFPE Tissue LEV DNA Purification Kit as the method of choice for isolating genomic DNA from FFPE samples. This method provides the highest yield and highly concentrated samples. The additional time saving is critical for the quality control of our off-the-shelf FFPE reference standards as well as our custom reference standards used in this study.
An additional advantage of the Maxwell platform is the reduced risk of contamination, which is essential for control of our manufacturing purposes. Complementary to our FFPE reference standards we also provide genomic DNA reference standards extracted from our isogenic cell lines. The risk of cross-contamination has to be controlled and reduced to an absolute minimum, and the best system we have evaluated is the Maxwell Cell DNA Purification Kit.
