- Products
- Dharmaconスクリーニングライブラリー
Mouse siGENOME siRNA Library - Epigenetics
Trusted for superior silencing since 2002

The siGENOME Mouse Epigenetics siRNA Library targets enzyme classes characterized to play a role in epigenetic mechanisms (see Reference 3). These inheritable factors that regulate genetic expression are of growing importance to scientific understanding of disease progression and trait inheritance. Modification of histones, such as methylation or acetylation, plays a key role in epigenetics as the extent to which DNA is wrapped around these protein complexes will alter the availability of genes in those DNA segments to be activated. siGENOME siRNA reagents are globally recognized and trusted for highly effective performance in RNAi screening applications. Our expertise in siRNA design, experimental optimization, and siRNA screening strategies provide end-to-end support of your screening efforts. The Epigenetics library targets genes which encode histone and DNA modification factors like methyltransferases, acetyltransferases, kinases and deacetylases, in addition to genes with a role in chromatin remodeling and histone ubiquitination.
Highlights
- Guaranteed target knockdown (see Specifications tab)
- Antisense strand loading into RISC ensured by thermodynamic analysis and selective application of a sense strand-blocking modification (ON-TARGET)
- Available as SMARTpool or Set of 4 siRNA reagents arrayed in 96-well plates
Gene Targets
For a complete list of target genes in this siRNA Library, please contact Scientific Support or your local Sales Representative.
Our siRNA knockdown guarantee
siGENOME and ON-TARGETplus siRNA reagents (SMARTpool and three of four individual siRNAs) are guaranteed to silence target gene expression by at least 75% at the mRNA level when demonstrated to have been used under optimal delivery conditions (confirmed using validated positive control and measured at the mRNA level 24 to 48 hours after transfection using 100 nM siRNA).
Note: Most siGENOME and ON-TARGETplus siRNA products are highly functional at 5 to 25 nM working concentration.
Screening with siGENOME SMARTpool siRNA reagents provides effective target silencing.
Effective silencing achieved for 440 kinase genes when screened with SMARTpool siRNA reagents in two cell lines. Under screening conditions these overall results provide validation of siRNA design and SMARTpool technology for effective target knockdown. HeLa and MCF10a cells were transfected with 50 nM of each siRNA using DharmaFECT 1 transfection reagent. Target mRNA levels were determined by branched DNA assay (Panomics Quantigene® Reagent System). The pie chart represents the summary of silencing achieved for the entire data set (880 total data points). The bar graph is a representative sample of genes assayed.
Unnecessary sense-strand inactivation can increase off-target activity
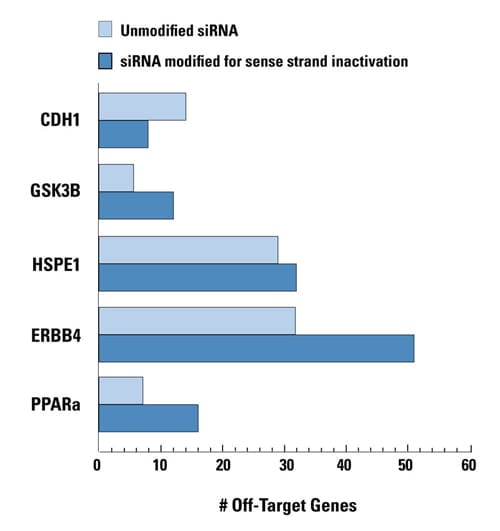
Unmodified and sense strand-inactivated siRNAs were used to target five genes. In four cases, off-targets were increased due to enhanced RISC loading of the antisense strand when the sense strand was modified. The unmodified siRNAs have natural guide-strand loading characteristics. All siRNAs had comparable silencing potency. Data shown represents genes down-regulated by twofold or more. HEK293 cells were transfected with 100 nM siRNA using 0.2 μL of DharmaFECT 1. Data was analyzed at 24 hours by genomewide microarray analysis (Agilent).
Why not modify ALL siGENOME siRNAs to ensure proper strand loading? It has been demonstrated by Dharmacon scientists1 and others2 that forcing antisense (guide) strand entry into RISC may actually INCREASE off-targets due to increased loading of the guide strand and resulting off-target activity by its seed region. siGENOME siRNAs are designed with thermodynamic properties to naturally facilitate guide strand entry to RISC, which has been demonstrated to correlate with functionality1. However, in cases where a high-scoring siGENOME siRNA does not possess ideal strand-loading characteristics, a sense (passenger) strand-inhibiting chemical modification (ON-TARGET) is utilized to promote guide strand entry. Approximately 20% of siGENOME siRNAs carry the ON-TARGET modification.
- B. D. Parsons et al., A direct phenotypic comparison of siRNA pools and multiple individual duplexes in a functional assay. PLoS One. 4(12), e8471 (29 December 2009).
- M. Jiang et al., Tales from an academic RNAi screening facility; FAQs. Brief Funct Genomics. 10(4), 227-237 (Epub 28 April 2011, July 2011). [doi: 10.1093/bfgp/elr016]
- J. Comley. Epigenetics: An emerging target class for drug screening. Drug Discovery World. Spring 2011, 40-55 (2011).
- J. D. Zhang et al., Time-resolved human kinome RNAi screen identifies a network regulating mitotic-events as early regulators of cell proliferation. PLoS One. 6(7), e22176 (Epub 13 July 2011).
- J. A. Smith et al., Genome-wide siRNA screen identifies SMCX, EP400, and Brd4 as E2-dependent regulators of human papillomavirus oncogene expression. Proc. Natl. Acad. Sci. U S A. 107(8), 3752-3757. (Epub 2 February 2010, 23 February 2010).
- A. Genovesio et al., Visual genome-wide RNAi screening to identify human host factors required for Trypanosoma cruzi infection. PLoS One. 6(5), e19733 (Epub 20 May 2011).
- G. Hu et al., A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 23(7), 837-848 (1 April 2009).
- R. D. Paulsen et al., A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 35(2), 228-239 (31 July 2009).
- M. Tsui et al., An intermittent live cell imaging screen for siRNA enhancers and suppressors of a kinesin-5 inhibitor. PLoS One. 4(10), e7339 (5 October 2009).
- A. L. Brass et al., Identification of host proteins required for HIV infection through a functional genomic screen. Science. 319(5865), 921-926. (Epub 10 January 2008, 15 February 2008).
- C. Swanton et al., Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 11(6), 498-512 (11 June 2007).
- A. W. Whitehurst et al., Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 446(7137), 815-819 (12 April 2007).
