Research conducted by Jian-Hua Luo, M.D., Ph.D. of the Pittsburgh School of Medicine marks the first time gene editing has been used to specifically target cancer fusion genes1.
These hybrid genes, formed from two previously separate genes, produce abnormal proteins that can be a catalyst to faster and/or further cancer growth. In patients this causes far more invasive cancers, reducing life expectancy and survival chances, so successful gene knockout offers many potential therapeutic applications.
Targeting cancer genes
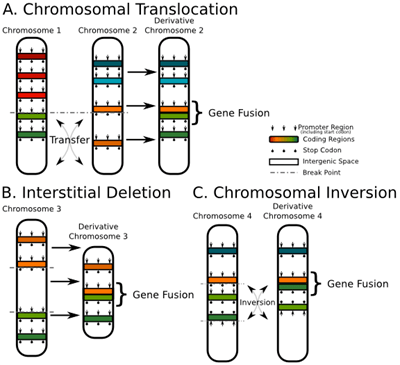
The team used CRISPR gene editing to target DNA sequences unique to these fusion genes, delivered by a pair of adenoviruses, one designed to deliver the CRISPR machinery and the guide RNA to direct it to the breakpoint, and the second to deliver a DNA sequence designed to insert an EGFP-HSV1-tk construct into the breakpoint. When both viruses were present, a vulnerability was introduced that lead to (ganciclovir-mediated) cell death.
Since these target mutant genes only exist in the cancer cells, any therapy based on this technique will be less likely to have side effects associated with traditional cancer treatments such as chemotherapy which affects both healthy and cancer cells.
Testing with research models of cancer
Using xenograft animal research models, over an 8-week treatment period the team demonstrated that the fusion gene targeting resulted in a 30% tumor size reduction, forty times smaller than a control group and also reducing the spread of the cancer, in fact, almost all mouse subjects treated with the new approach survived.
Dr Luo explained "Other types of cancer treatments target the foot soldiers of the army. Our approach is to target the command center, so there's no chance for the enemy's soldiers to regroup in the battlefield for a comeback."
Study
Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene
