3D culturing is a valuable and increasingly popular cell culture technique. This model recapitulates some physiological characteristics of tissues and tumors driven by cell-cell and cell-matrix contacts, circumventing the need for in vivo experiments in some cases.
Achieving strong target knockdown in 3D cultures via lipid mediated transfection reagents can be a challenging endeavor, caused by limited access of transfection reagent: siRNA complexes to the interior cell layers. Knockdown inefficiency can result from the proliferative outer layers of the spheroid preventing the transfection reagent mixture from reaching inner cellular layers or from failure of the transfection reagent: siRNA complex to diffuse through the extracellular matrix in scaffold-based culture models. Our self-delivering Accell siRNA technology – originally designed for difficult-to-transfect cells – can be a helpful tool for researchers aiming to regulate gene expression in a 3D cell culture model. Here, we recommend some guidelines and pre-requirements to establish reliable Accell siRNA delivery conditions in 3D spheroid cultures:
- Suitable cells according to your study background: check out the literature for spheroid forming cells
- Preliminary culture conditions: evaluate required scaffolds (matrices), plate formats, seeding densities, spheroid morphology and size, culture duration, and viability (stress/cell death). Perform preliminary culturing experiments in serum free Accell siRNA Delivery Media or in reduced serum culture media and compare viability and proliferation to cells cultured in their regular cell culture media. During Accell siRNA exposure, serum may interfere with Accell siRNA delivery to cells
- Initial knockdown experiments: use positive- and negative Accell siRNA controlsand quantify mRNA down-regulation by RT-qPCR. You may need to test higher siRNA concentrations than the recommended Accell end concentration of 1 µM in 2D cultures to achieve strong knockdown. For long term cultures, replenishment of Accell siRNA might also be necessary to increase and/or prolong the knockdown efficiency
Figure 1 demonstrates a knockdown experiment in HCT-116-derived spheroids. During the first 4 days, HCT-116 cells form spheroids. Then, at day 4 and 6, spheroids were treated with Accell siRNAs targeting either MAP2K1, MAP2K2 or both. Next, spheroids were harvested, and mRNA was isolated. Using Dharmacon´s Accel siRNA, a 60-80% specific target knockdown is achieved in difficult-to-penetrate 3D cultures.

Figure 1A) Dosing timeline for Accell siRNA delivery on spheroids formed in a 96-well Ultra-Low Attachment (ULA) microplate1.
2,500 HCT-116 cells per well were seeded in 100 µl HCT-116 standard growth medium2, 3 biological replicates per condition. The plate was incubated at 37 °C 5% CO2 for 4 days to form spheroids. Accell siRNA was then delivered at 1 µM diluted in either HCT-116 medium with 0% fetal bovine serum or in Accell medium. Spheroids were incubated for 48 hours at 37 °C 5% CO2 before replenishing with a second dose of 1 µM Accell siRNA and incubated for an additional 48 hours. Next, the mRNA was extracted, and RT-qPCR was performed to confirm knockdown.3
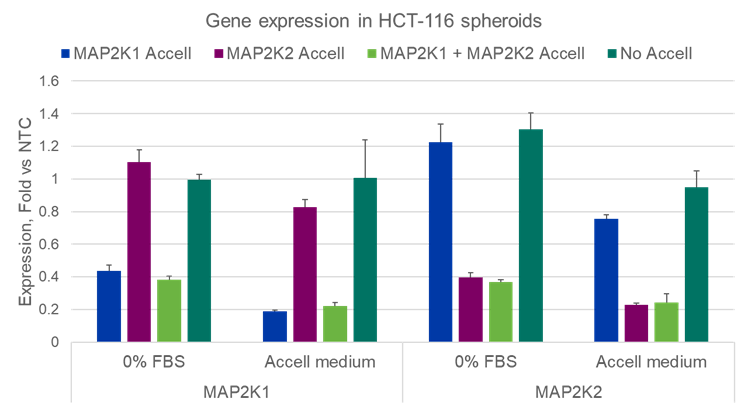
Figure 1B) High knockdown efficiency and target specificity in 3D cultures with Accell siRNA.
RT-qPCR data showing target specific knockdown of MAP2K1 and MAP2K2 genes with Accell siRNA in HCT-116 spheroids formed in a 96-well ULA microplate1.
Footnotes
- CellCarrier Spheroid ULA 96-well Microplates (Revvity, Cat.: 6055330)
- Optimization necessary for cell line, plate type, and final Accell concentration for best knockdown
- Figure created with BioRender.com
Check out our Accell siRNA reagents for your next Accell siRNA 3D culture experiments and contact Revvity´s Scientific Support.
