- CRISPR activation reagents
- MS2 tracrRNA
CRISPRa MS2 tracrRNA
Synthetic trans-activating CRISPR RNA compatible with the SAM transcriptional activation system

For gene activation with the synergistic activation mediator (SAM) system, CRISPRa MS2 tracrRNA (formerly called SAM tracrRNA) can be used with CRISPRa synthetic crRNA for gene activation studies. MS2 tracrRNA is a chemically synthesized and HPLC-purified long RNA molecule based on the optimized published S. pyogenes tracrRNA sequence (Jinek, 2012) that also contains an MS2 aptamer sequence that binds to the MS2-p65-HSF1 component of the SAM system (Konermann et al., 2015).
Requirements for gene activation in mammalian cells using the SAM system:
- A stable cell line expressing both components (NLS-dCas9-VP64 and MS2-p65-HSF1) of the SAM activation system
- CRISPRa MS2 tracrRNA
- CRISPRa synthetic crRNA designed to the gene target site of interest
How much crRNA & tracrRNA do I need?
This table provides the approximate number of experiments that can be carried out for lipid transfection methods at the recommended crRNA:tracrRNA working concentration (25 nM:25nM) in various plate/well formats. Calculations do not account for pipetting errors. For crRNA libraries, use (# wells)*(nmol per well) to determine the approximate amount of tracrRNA required. For instance, a 100 well library at 0.5 nmol per well would require 50 nmol of tracrRNA. The bulk sizes of tracrRNA are recommended for large library projects.
| crRNA nmol | tracrRNA nmol | 96-well plate 100 µL reaction volume | 24-well plate 500 µL reaction volume | 12-well plate 1000 µL reaction volume | 6-well plate 2500 µL reaction volume |
|---|---|---|---|---|---|
| 2 | 2 | 800 | 160 | 80 | 32 |
| 5 | 5 | 2000 | 400 | 200 | 80 |
| 10 | 10 | 4000 | 800 | 400 | 160 |
| 20 | 20 | 8000 | 1600 | 800 | 320 |
Transcriptional activation of gene targets in SAM system expressing cell lines
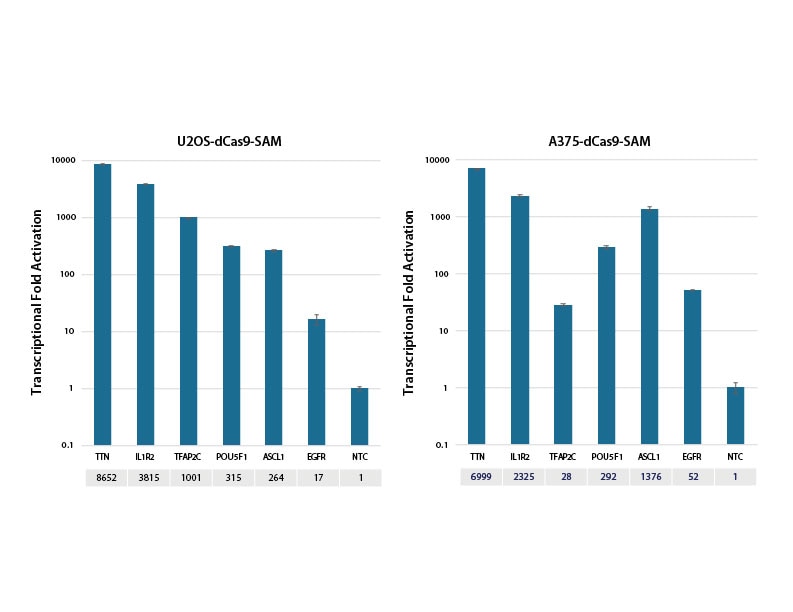
U2OS and A375 cells stably expressing dCas9-VP64 and MS2-p65-HSF1 (dCas9-SAM) were plated at 10,000 cells/well and transfected using DharmaFECT 4 (U2OS) or DharmaFECT 1 (A375) Transfection Reagent with synthetic crRNA:tracrRNA (25nM). Four predesigned crRNAs targeting each gene were pooled (to a total concentration of 25nM). Cells were harvested 72 hours post-transfection and the relative gene expression was calculated using RT-qPCR. The relative expression of each gene was calculated with the ΔΔCq method using PPIB as the housekeeping gene and normalized to a non-targeting control.
Transcriptional activation of gene targets in U2OS-dCas9-SAM cell lines: individual and pooled crRNA
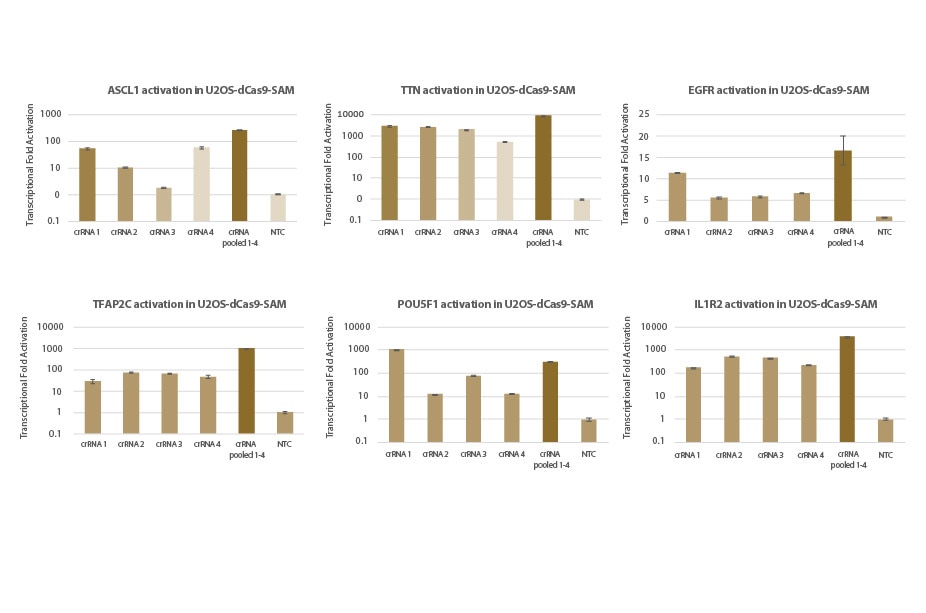
U2OS cells stably expressing dCas9-VP64 and MS2-p65-HSF1 (dCas9-SAM) were plated at 10,000 cells/well and transfected using DharmaFECT 4 Transfection Reagent with synthetic crRNA:tracrRNA (25nM). Four predesigned crRNAs targeting each gene were transfected individually or pooled (to a total concentration of 25nM). Cells were harvested 72 hours post-transfection and the relative gene expression was calculated using RT-qPCR. The relative expression of each gene was calculated with the Δ Δ Cq method using PPIB as the housekeeping gene and normalized to a non-targeting control.
Transcriptional activation of gene targets in A375-dCas9-SAM cell lines: individual and pooled crRNA
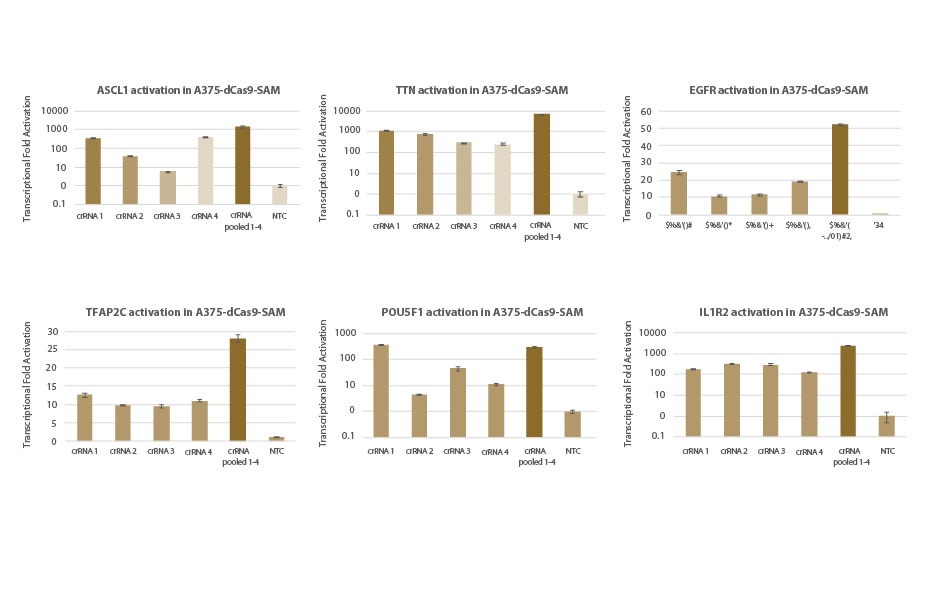
A375 cells stably expressing dCas9-VP64 and MS2-p65-HSF1 (dCas9-SAM) were plated at 10,000 cells/well and transfected using DharmaFECT 1 Transfection Reagent with synthetic crRNA:tracrRNA (25nM). Four predesigned crRNAs targeting each gene were transfected individually or pooled (to a total concentration of 25nM). Cells were harvested 72 hours post-transfection and the relative gene expression was calculated using RT-qPCR. The relative expression of each gene was calculated with the Δ ΔCq method using PPIB as the housekeeping gene and normalized to a non-targeting control.
CRISPRa SAM workflow
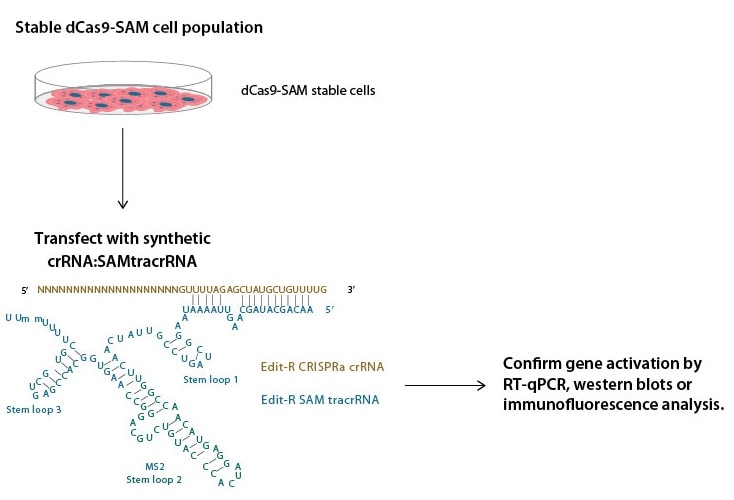
Horizon's CRISPRmod MS2 tracrRNA (also called SAM tracrRNA) contains an MS2 aptamer on stem loop 2 that recruits MCP-p65-HSF1. Combined with a catalytically inactivated Cas9-VP64 and a gene specific CRISPRa crRNA, this utilization of the synergistic activation mediator (SAM) system achieves robust transcriptional activation.
