- CRISPR activation reagents
- CRISPRmod CRISPRa Synthetic sgRNA
CRISPRmod CRISPRa Synthetic sgRNA
Predesigned synthetic guide RNA for over-expression of human and mouse genes using CRISPR activation. Just search for your gene! Available as pooled or individual reagents.
Genome-wide human and mouse synthetic sgRNA reagents are specially designed for CRISPRa. In conjunction with dCas9-VPR expression, you can harness the power of the CRISPR-Cas9 system for activation of your favorite gene.
1Start Here
2Choose
Endogenous gene activation with an easy-to-use synthetic reagent

The CRISPR activation (CRISPRa) system is a unique adaptation of the classical CRISPR-Cas9 gene editing system which utilizes a catalytically deactivated Cas9 (dCas9)that is fused to one or more transcriptional activators. When paired with a well-designed guide RNA that targets a gene near a promoter region, or transcriptional start site (TSS), it promotes transcriptional activation.
Review our CRISPRa application page to get an overview of the technology!
Highlights of CRISPRa sgRNA reagents
- Single guide RNA (sgRNA) design streamlines protocols with similar robust activation to two part crRNA:tracrRNA reagents
- Available as individual reagents or a pool of three sgRNAs to provide highly effective transcriptional activation (See Supporting Data tab).
- Designs from a published algorithm by Horlbeck, et. al. that demonstrates strong levels of gene activation from optimized designs (See References tab).
- Chemically modified sgRNAs provide additional stability against nuclease degradation and improve overall performance.
- For genes with alternative characterized start sites, distinct guide RNA designs are available (labeled P2).
Required components for CRISPRa gene activation using synthetic sgRNA
- A lentiviral expression plasmid, lentiviral particles or mRNA for dCas9-VPR; a mammalian codon-optimized S. pyogenes deactivated Cas9 fused to VPR activation domains (also compatible with SunTag technology).
- A predesigned CRISPRa sgRNA targeting the gene of interest.
CRISPRa workflow diagram with stable dCas9-VPR expression

CRISPR activation workflow with Lentiviral dCas9-VPR and synthetic crRNA:tracrRNA (right side) or Lentiviral expressed sgRNA (left side).
CRISPRa controls
CRISPRa synthetic sgRNA positive controls
- CRISPRa sgRNAs targeting well-characterized genes to determine the effectiveness of your experimental conditions for maximum activation.
CRISPRa synthetic sgRNAs non-targeting controls
- Non-targeting controls to evaluate baseline cellular responses to CRISPRa components in the absence of gene target-specific sgRNA.
CRISPRa related products
CRISPRa dCas9-VPR
- Lentiviral particles, purified plasmid or mRNA that express nuclease-deactivated Cas9 fused to transcriptional activators. When complexed with a guide RNA will trigger an endogenous gene’s expression.
Tris Buffer pH7.4
- Dharmacon 10 mM Tris-HCl Buffer is recommended for resuspension of any single-strand synthetic RNA, such as CRISPRa sgRNAs.
DharmaFECT transfection reagents
- For efficient siRNA & CRISPR guide RNA delivery
How much CRISPRa sgRNA do I need?
This table provides the approximate number of experiments that can be carried out for lipid transfection methods at the recommended sgRNA working concentration (25 nM) in various plate/well formats using either a pool or individual sgRNA. Calculations do not account for pipetting errors.
| sgRNA nmol | 96-well plate 100 µL volume | 24-well plate 500 µL volume | 12-well plate 1000 µL volume | 6-well plate 2500 µL volume |
|---|---|---|---|---|
| 2 | 800 | 160 | 80 | 32 |
| 5 | 2000 | 400 | 200 | 80 |
| 10 | 4000 | 800 | 400 | 160 |
| 20 | 8000 | 1600 | 800 | 300 |
Immunofluorescent analysis demonstrates effective CRISPRa gene activation

U2OS-dCas9-VPR stable cells were plated on Phenoview imaging plates at 10,000 cells/well and transfected using 0.2 uL/well DharmaFECT 1 Transfection Reagent with 25 nM individual or pooled synthetic single guide RNA (sgRNA) targeting POU5F1, or non-targeting control (NTC). 72 hours post-transfection, cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 in PBS and blocked in 3% bovine serum albumin (BSA) and 0.5% Triton-X 100 in PBS, followed by incubation with target-specific antibody and fluor-conjugated secondary antibody and Hoeschst 33342. Images were generated on an Operetta CLS High-Content Analysis system.
Individual CRISPRa sgRNAs achieve robust target gene activation as individual or pooled reagents
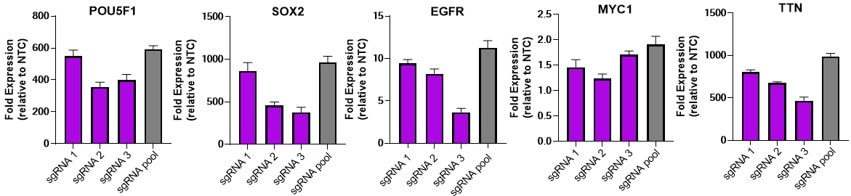
U2OS cells stably expressing integrated CRISPRa dCas9-VPR were plated at 10,000 cells/well and transfected using DharmaFECT 1 Transfection Reagent (0.2 uL/well) with 25 nM CRISPRa single or pooled synthetic single guide RNA (sgRNA) targeting POU5F1, SOX2, EGFR, MYC1 or TTN. Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
Individual CRISPRa crRNAs achieve robust target gene activation on their own, or when pooled together in a single reagent
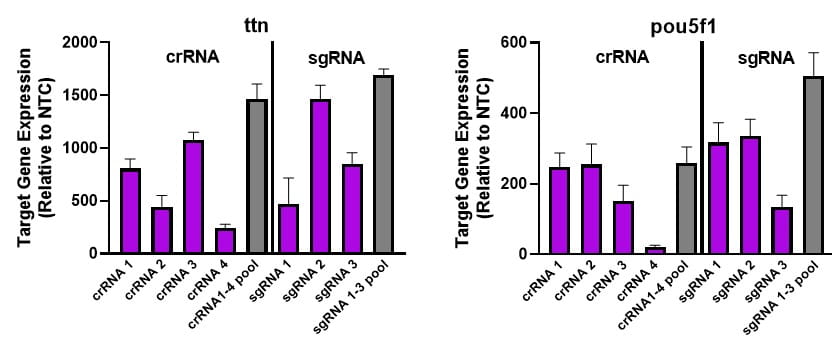
NIH-3T3 cells stably expressing integrated CRISPRa dCas9-VPR (driven by mCMV promoter) were plated at 10,000 cells/well and transfected using DharmaFECT 1 Transfection Reagent (0.2 uL/well) with CRISPRa synthetic single guide RNA (sgRNA) targeting ttn or pou5f1 genes. Four CRISPRa sgRNAs were used either individually or pooled (to an individual or pooled concentration of 25 nM). Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
Gene activation using CRISPRa synthetic single guide RNA in dCas9-VPR stable human and mouse cell lines

Human U2OS or mouse NIH-3T3 cells stably expressing integrated CRISPRa dCas9-VPR (driven by hEF1a promoter in human and mCMV promoter in mouse cells) were plated at 10,000 cells/well and transfected using DharmaFECT 1 Transfection Reagent (0.2 uL/well) with 25 nM individual or pooled CRISPRa synthetic single guide RNA (sgRNA) targeting either human (TTN or POU5F1) mouse (pou5f1 or ttn) gene targets. Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
sgRNA dose response curve demonstrates effective gene activation at 25 mM sgRNA concentration
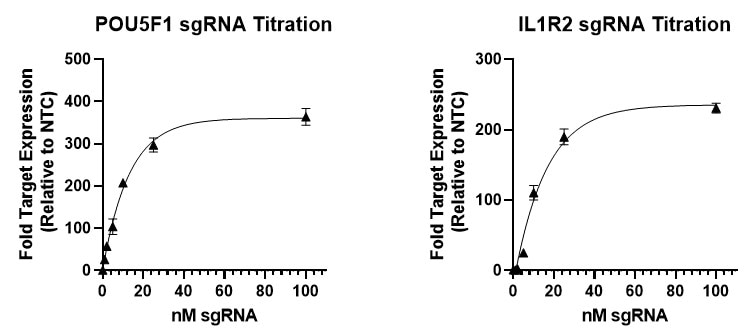
U2OS-dCas9-VPR stable cells were plated at 10,000 cells/well and transfected using 0.2 uL/well DharmaFECT 1 Transfection Reagent with CRISPRa synthetic single guide RNA (sgRNA) targeting POU5F1 or IL1R2 at six concentrations (0, 1, 5, 10, 25 and 100 nM). At 72 hours post-transfection, cell lysates were used to generate total cDNA, from which target gene expression was assessed using RT-qPCR. Relative expression of each gene was calculated with the Cq method using beta-actin mRNA as the housekeeping gene and normalized to a non-targeting control. Data points were fit to a one phase exponential decay equation.
CRISPRa gene activation fold depends on the endogenous gene expression level
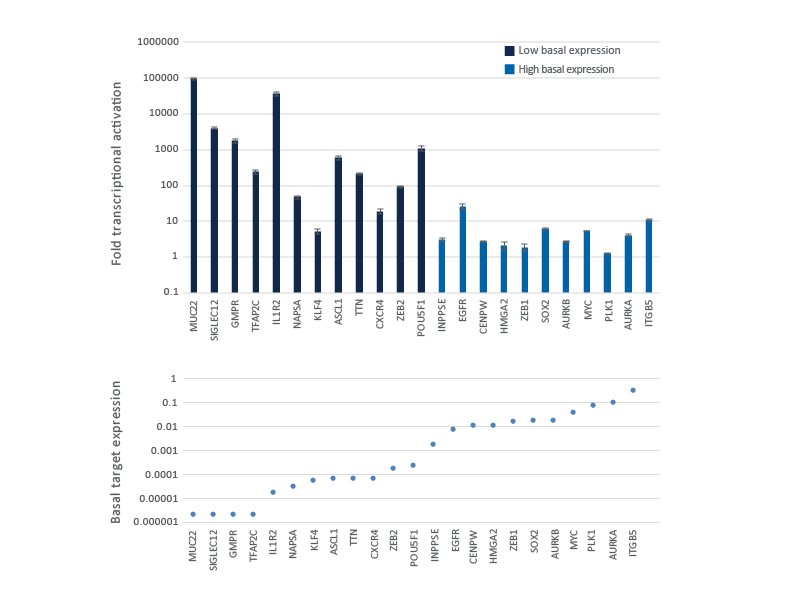
Genes with low or no basal expression are easier to activate to a robust level, while genes already expressed at high level are more difficult to further over-express. U2OS-dCas9-VPR stable cells were plated at 10,000 cells/well and transfected using DharmaFECT 4 Transfection Reagent with CRISPRa synthetic crRNA:tracrRNA pools (25 nM) targeting 23 genes with different basal level of expression. Cells were harvested 72 hours post-transfection and the relative gene expression was measured using RT-qPCR. The CRISPRa-mediated fold transcriptional activation is shown in the upper graph where the genes are ordered from low to high level of basal transcript expression in samples treated with NTC control and is shown in the lower graph as basal target gene expression (compared to GAPDH control).
CRISPRa gene activation is observed at 24 hours and peaks at 48-72 hours
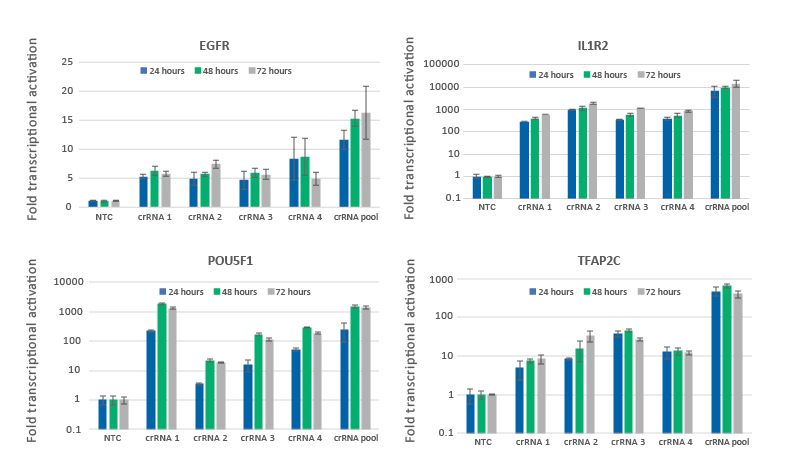
U2OS-dCas9-VPR stable cells were plated at 10,000 cells/well and transfected using DharmaFECT 4 Transfection Reagent with CRISPRa synthetic crRNA:tracrRNA targeting EGFR, IL1R2, POU5F1 or TFAP2C. Four CRISPRa crRNAs were used either individually or pooled (to a total concentration of 25 nM). Cells were harvested at 24, 48, and 72 hours post-transfection and the relative gene expression was measured using RT-qPCR. The relative expression of each gene was calculated with the Cq method using GAPDH as the housekeeping gene and normalized to a non-targeting control.
Robust CRISPRa gene activation peaks between 24 to 72 hours
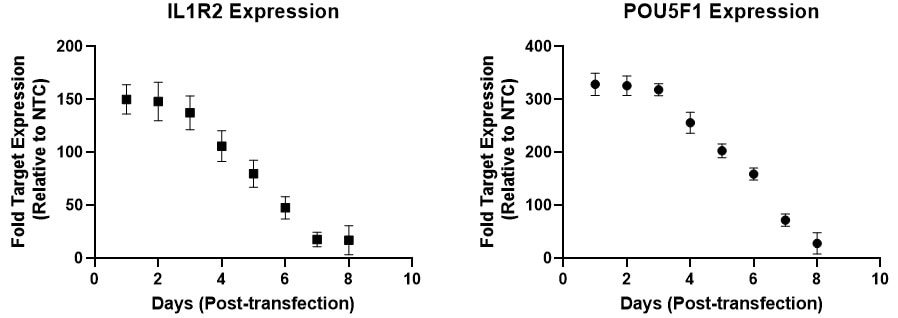
U2OS-dCas9-VPR stable cells were plated at 5,000 cells/well and transfected using 0.2 uL / well DharmaFECT 1 Transfection Reagent with 25 nM CRISPRa synthetic single guide RNA (sgRNA) targeting POU5F1, IL1R2, or non-targeting control (NTC). Transfected cells were passaged every 2-3 days to avoid overconfluency. Cells were harvested at 72 hours post-transfection and gene expression was assessed using RT-qPCR. Relative fold transcriptional activation for each gene was calculated with the Cq method using beta-actin as the housekeeping gene and normalized to an experiment using non-targeting control sgRNA.
