Mimix NIPT reference standards
Patient-like non-invasive prenatal testing (NIPT) reference material
Revvity has developed Mimix™ NIPT reference standards - a range of matched maternal-fetal reference material to assist researchers to confidently verify and control molecular research workflows for fetal aneuploidies and other chromosomal abnormalities from maternal blood samples.
Covering the most commonly tested for aneuploidies, trisomy 13, trisomy 18 and trisomy 21, these control materials can support the verification, monitoring and troubleshooting of a broad range of prenatal assay methods.
The importance of NIPT reference standards
NIPT involves screening for fetal abnormalities detected in maternal plasma samples and serve as a non-invasive screening method, compared to invasive Amniocentesis and Chorionic Villus Sampling (CVS), bearing a risk of procedural miscarriage.
In maternal blood, detecting these abnormalities from small amounts of fragmented cell-free DNA (cfDNA) in a high background of maternal cfDNA is a challenge. Conclusively, there is a need to systematically ensure that the NIPT results are consistent and precise.
Revvity’s reference standards help scientists check that screening tests for Trisomy 13 (Patau syndrome), Trisomy 18 (Edwards syndrome), and Trisomy 21 (Down syndrome) and Euploid detection are performing as expected.
Mimix NIPT reference standard applications
- Analyze the analytical sensitivity and accuracy of prenatal assays
- Verify, monitor and troubleshoot prenatal genetic research assays
- Fetal fractions designed to mimic maternal-fetal samples to establish assay baseline
- Support end-to-end research workflows
Our NIPT reference standards have been extensively tested using ddPCR and Vanadis™ NIPT systems** to:
- Support individual selection for research studies
- Assess therapeutic efficacy
- Control end-to-end verification of workflows
- Reliably verify research workflows using matched maternal-fetal cfDNA based reference material
- Provide comparable, repeatable results to reduce misinterpretation of data
Why choose Mimix reference standards?
- Cell line-derived controls for the closer representation of maternal blood samples
- Sustainable source of matched maternal-fetal cfDNA in synthetic plasma
- Reproducible controls at 40 ng/mL concentration, 170 bp size distribution and 10% fetal fraction
- Manufactured under ISO 9001:2015
Vanadis system results
The Vanadis NIPT system** enables researchers to conduct targeted cfDNA analysis allowing the quantification of chromosomes 21, 18, 13 without PCR, sequencing, microarrays or microfluidics. Instead, 3500 cfDNA fragments per chromosome are captured by highly specific fluorophores are incorporated for each chromosome being analyzed. A proprietary nanofilter plate then captures the labeled DNA molecules for imaging to determine a density score.

*Average normalized ratios per product
Table showing representative data generated on the Vanadis NIPT system ** using Revvity’s Mimix NIPT reference standards. Normalized ratios are calculated using the Vanadis NIPT system** imaging data and density scores for chromosomes 13, 18 and 21, which in turn are used to determine Z-scores for each.
 |
 |
 |
Normalized ratio Representative normalized ratio plots using data generated on the Vanadis NIPT system** and Mimix NIPT reference materials. Both the chromosome being analyzed (13, 18 or 21) and euploid data points should lie on or close to the diagonal, with the euploid being close to the center of the plot and the chromosome being analyzed in the upper right quadrant if positive for the aneuploidy.
 |
 |
 |
 |
Representative z score plots using data generated on the Vanadis NIPT system** and Mimix NIPT reference standards. All samples that result in a positive outcome for trisomy 13, 18 or 21 show z-scores above a threshold of 3.15 and/or 3.5.
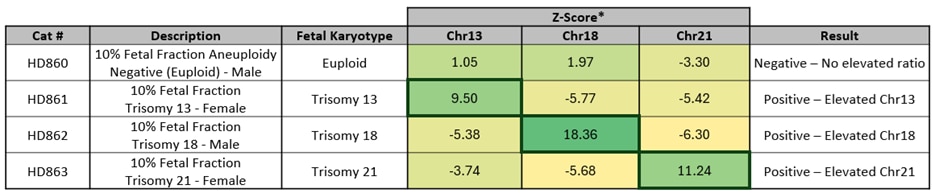
*Average Z-Scores per product
*Mimix reference standards are for research use only. Not for use in diagnostic procedures. Vanadis products may not be licensed in accordance with the laws in all countries, such as the United States and Canada. Please check with your local representative for availability.
**Vanadis products may not be licensed in accordance with the laws in all countries, such as the United States and Canada. Please check with your local representative for availability.
Order NIPT Products
These cfDNA reference standards mimic the three most common chromosomal aneuploidies. Each of the product is based on a unique matched maternal-fetal set of cell lines.
Euploid Male-Matched Reference Standard
This control is negative for Trisomy 13, 18 and 21 aneuploidies, providing matched maternal-fetal cfDNA, isolated from a healthy patient.
Helpful resources
Vanadis NIPT System
The Vanadis NIPT system is a cell-free DNA analysis solution intended for chromosome quantification and serves to benefit laboratories with the easy to use, and scalable platform.
Imaging single DNA molecules for high precision NIPT
Dahl, F. et al. Imaging single DNA molecules for high precision NIPT. Sci Rep 8, 4549 (2018).
