- Dharmacon Screening libraries
- CRISPRmod CRISPRa All-in-one Lentiviral sgRNA Whole Genome Pooled Library
This library format is beneficial for performing gene activation in difficult-to-transfect or primary cells allowing for efficient genomic screening facilitating advancements in genetic research and therapeutic discovery.
The choice of promoters (mCMV and hEF1α) allows for optimal dCas9-VPR expression in the cells of interest.
CRISPRmod CRISPRa system requires two components to operate: a CRISPRa sgRNA and a catalytically deactivated Cas9 (dCas9) fused to transcriptional activators (VPR). Our CRISPRa all-in-one libraries provide a straightforward method to study genomic function by overexpression in its native context.
The CRISPRmod CRISPRa guide RNA designs use a published CRISPRa v2 algorithm (Horlbeck et al 2016). CRISPRa guide RNAs target sequences upstream of the target gene's promoter region or the transcriptional start site (TSS) to result in activation.
| Schematic map of the CRISPRmod CRISPRa All-in-one lentiviral vector |
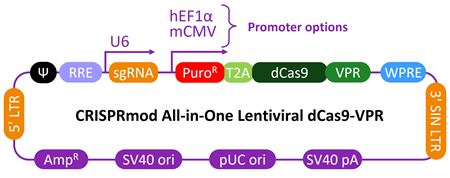 |
Highlights:
- Efficient All-in-one vector system utilizing single lentiviral vectors for both dCas9-VPR and gene-specific sgRNA expression
- Rationally designed lentiviral sgRNAs mediate efficient and specific gene activation
- Deep and broad coverage with 4 or 8 sgRNAs per gene across the human genome for increased hit confidence
- Multiple promoter options for robust activation in biologically relevant cell types
- High quality, concentrated, purified lentiviral particles for direct transduction with minimal cytotoxicity; delivered at titers of ≥ 2 x 107 TU/mL ( ± 20%)
Each CRISPRmod CRISPRa All-in-one lentiviral pooled screening library includes:
- ≥ 2 x 107 TU/mL (± 20%) lentiviral particles in 10 x 100 uL aliquots with mCMV or hEF1α promoter options
- Up to 100 non-targeting gRNA negative controls bioinformatically confirmed to not align with (target) any gene in the human genome
- A data file containing complete library information, including: gene annotations, sgRNA target sequences, complete list of controls, and counts per millions of mapped reads
Products recommended in our validated protocol:
- Vector-matched CRISPRa EGFP delivery controls for transduction optimization and promoter selection
- NEW: NGS Library Prep Kit (Recommended)
- Primer set options available for hEF1α and mCMV promoters and at two different sizes for 12 or 24 whole genome screens
- Designed to amplify and sequence sgRNA constructs from gDNA without bias
- One complete solution for sequencing on an Illumina® platform without the need for additional reagents
Information on Edit-R Lentiviral sgRNA pooled library coverage
The number of genes and constructs are subject to change at any time without notice. Clone and gene counts are available upon request. Contact Scientific Support
Volume of lentiviral particles required for whole genome screening
| Fold representation | Replicates | sgRNAs per gene | Volume of lentiviral particles |
|---|---|---|---|
| 200 | 2 | 4 | 1.7 mL |
| 200 | 2 | 8 | 3.3 mL |
| 400 | 2 | 4 | 3.3 mL |
| 400 | 2 | 8 | 6.6 mL |
| NGS Library Prep Kit Contents | Small: 12 Whole Genome Screens | Large: 24 Whole Genome Screens |
|---|---|---|
| NEXTFLEX® PCR Master Mix (green cap) | 3600 μL | 7200 μL |
| Dharmacon HIT Identification Primers (orange cap) | 300 μL | 600 μL |
| NEXTFLEX® PCR II Barcoded Primer Mix | 4 μL | 4 μL |
| Resuspension Buffer | 12 mL | 24 mL |
| Nuclease-free water | 8 mL | 16 mL |
| Cleanup Beads | 7 mL | 15 mL |
Gene overexpression screening workflow using the CRISPRmod CRISPRa All-in-one Lentiviral Pooled Library platform
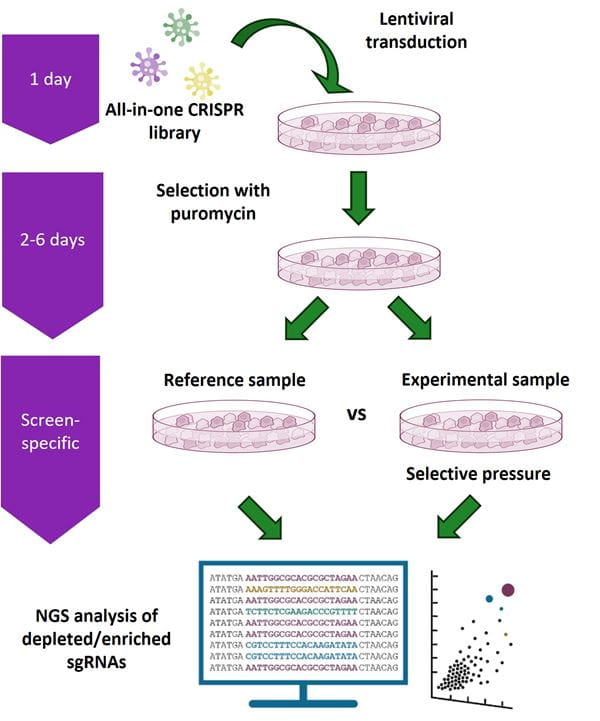
Cells are transduced at a low multiplicity of infection (MOI) with a CRISPRmod CRISPRa All-in-one Lentiviral Pooled Library and selected with puromycin. Transduced cells are split into reference and experimental populations for application of a selective pressure and/or phenotypic selection. Genomic DNA is then isolated from the reference and experimental populations of transduced cells and sgRNA constructs within the isolated gDNA are amplified using vector-specific NGS Library Prep Kits. Samples can then be multiplexed and sequenced on an Illumina® platform. Custom sequencing read primers are not required. The integrated sgRNA sequences in both reference and experimental samples are identified and relative abundance compared. sgRNA constructs that are enriched or depleted are identified as hits to be confirmed and studied further using individual CRISPRmod CRISPRa All-in-one Lentiviral sgRNAs in additional phenotypic and/or biochemical assays.
High quality pooled screening begins with rigorous lentiviral pooled library production
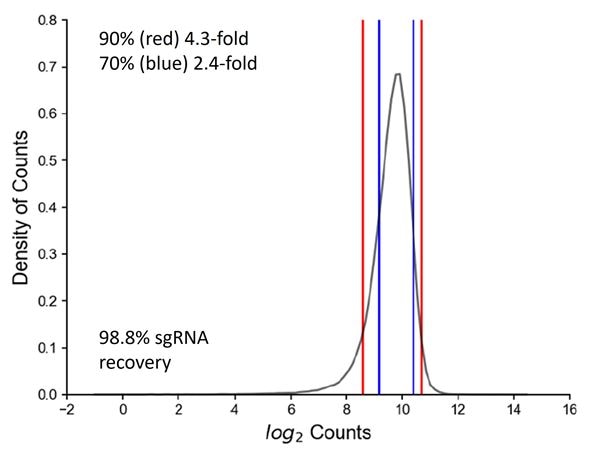
High quality pooled screening begins with rigorous lentiviral pooled library production. A CRISPRmod CRISPRa Human All-in-one Lentiviral Whole Genome Pooled Library comprising 4 lentiviral sgRNAs per gene was produced and the quality of the plasmid DNA library was verified by next-generation sequencing (NGS). Counts per million mapped reads were obtained to determine the percent-recovery of input sgRNAs (98.8%) and that the distribution of 90% and 70% of the sgRNAs in the pool are within 4.3- and 2.4- fold of each other, respectively, indicating high sgRNA recovery and uniform distribution of sgRNAs.
Matched fluorescent delivery controls enable rapid functional titering and selection of optimal promoter prior to screening
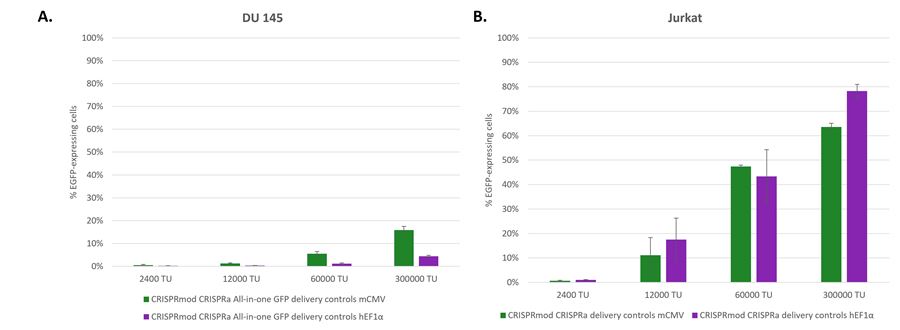
Matched fluorescent delivery controls enable rapid functional titering and selection of optimal promoter prior to screening. DU145 (A) and Jurkat (B) cells were transduced with 5-fold dilution series of CRISPRmod CRISPRa All-in-one GFP Delivery controls mCMV (green) and hEF1α (purple) with functional titering units (TU) derived in HEK293 cells. At 72 hours post-transduction, the percentages of EGFP-expressing cells were quantified by flow cytometry. Dilutions exhibiting between 5 to 25% EGFP-expressing cells can be used to calculate the relative functional titer in the cell line of interest and the relative transduction efficiency of the cell line. The percentages of EGFP-expressing cells or mean fluorescence intensity of EGFP-expressing cells at a given dilution (exhibiting 5-25% EGFP-expressing cells) can be used to compare promoter activity in the cell line. While both promoters are similarly active in Jurkat cells, the mCMV promoter is substantially more active in DU145 cells than the hEF1α promoter.
Highly concordant hits between whole-genome CRISPRa screens performed using the CRISPRmod All-in-one dCas9-VPR and Synergistic Activation Mediator (SAM) systems
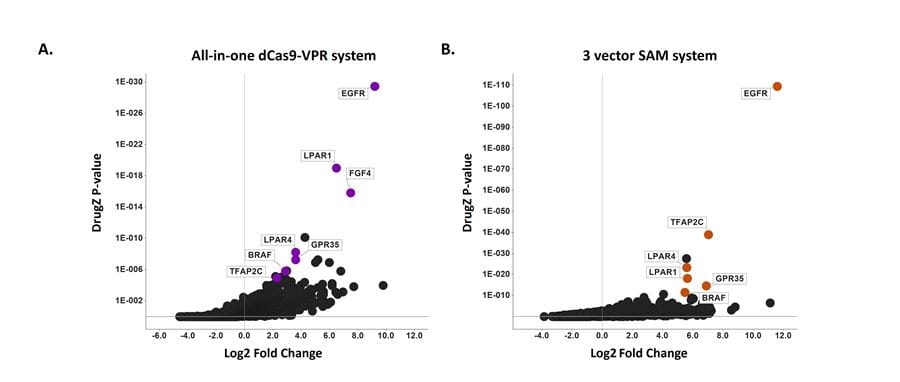
Highly concordant resistance hits using a CRISPRmod CRISPRa Human All-in-one lentiviral sgRNA Whole Genome Pooled Library (A) or the three vector Synergistic Activation Mediator System (B) in A375 melanoma cells treated with vemurafenib (PLX-4032) for 16 days. DrugZ screen analysis showing log2 fold enrichment of each gene and associated p-values; significantly enriched genes (positive Log2 Fold Change) drive resistance to the drug treatment indicating these genes potentially act as drug antagonists. Highlighted hits were identified with both technologies and have been previously validated by CRISPRa screening (Konermann et al., 2015).

