- Dharmacon Screening libraries
- Human ON-TARGETplus siRNA Library - Tyrosine Kinases
Human ON-TARGETplus siRNA Library - Tyrosine Kinases
siRNA designed and modified for greater specificity

With the Human ON-TARGETplus Tyrosine Kinase siRNA Library, researchers can suppress the expression of receptor and non-receptor tyrosine kinases critical for the control of several cell processes, including cell cycle, shape, proliferation, and differentiation. Tyrosine kinases constitute a large family of evolutionarily conserved enzymes that activate and deactivate a number of different metabolic pathways by catalyzing the phosphorylation of specific tyrosine residues on key effector proteins. Tyrosine kinases are typically classified into membrane-bound or non-membrane-bound groups and are found in nearly every mammalian cell type.
Highlights
- Patented dual-strand ON-TARGETplus modification pattern on all siRNAs to reduce off-targets
- Sense strand is modified to prevent interaction with RISC and favor antisense strand uptake
- Antisense strand seed region is modified to destabilize off-target activity and enhance target specificity
- Available as SMARTpool siRNA reagents or a Set of 4 siRNAs in 96-well plates
- Guaranteed target gene knockdown (see Specifications tab)
Highlights
- Six ready-to-use 96-well plate sets provided for two triplicate screens
- Pre-plated, validated RNAi controls included
- No aliquoting necessary, just resuspend and add cells
- siRNA reagents provided in clear plates at 6.25 pmol per well (50 nM final screening concentration)
- Black or white clear-bottom plates available to support assays involving fluorescent or luminescent detection
Gene targets
For a complete list of target genes in this siRNA Library, please contact Scientific Support or your local Sales Representative.
Our siRNA knockdown guarantee
siGENOME and ON-TARGETplus siRNA reagents (SMARTpool and three of four individual siRNAs) are guaranteed to silence target gene expression by at least 75% at the mRNA level when demonstrated to have been used under optimal delivery conditions (confirmed using validated positive control and measured at the mRNA level 24 to 48 hours after transfection using 100 nM siRNA).
Note: Most siGENOME and ON-TARGETplus siRNA products are highly functional at 5 to 25 nM working concentration.
False phenotypes due to off-targets are alleviated by ON-TARGETplus SMARTpool reagents while target gene knockdown is maintained
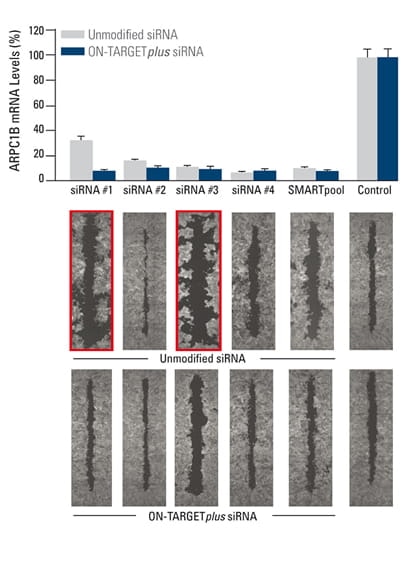
The effect of silencing ARPC1B on cell migration was studied in a breast cancer cell line. A monolayer of cells was uniformly scraped and the rate of cell migration to close the scrape (wound healing) was evaluated. Both unmodified and ON-TARGETplus siRNA reagents induced potent target knockdown. Inconsistent phenotypes due to off-target effects (red outline), were observed for cells transfected with unmodified individual siRNAs. The unmodified SMARTpool improved the false phenotype considerably, while the ON-TARGETplus SMARTpool significantly reduced off-target effects to produce a consistent phenotype. In collaboration with Kaylene Simpson, Laura Selfors, and Joan Brugge, Harvard Medical School.
ON-TARGETplus modifications reduce the overall number of off-targets and pooling reduces them even further
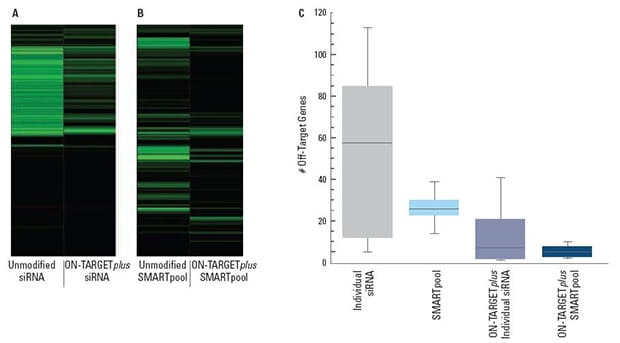
(A) and (B) are representative examples of off-target signatures with and without application of ON-TARGETplus modifications to (A) a single siRNA and (B) a SMARTpool reagent. Green bars indicate genes with 2-fold or more reduction of expression when treated with the indicated siRNA reagent. The ON-TARGETplus modifications reduced the off-targets when compared to unmodified siRNA. Pooling of siRNA and the ON-TARGETplus modification pattern independently, and in combination, provide significant reduction in off-target gene silencing. Panel (C) represents quantitation of off-targets (down-regulated by 2-fold or more) induced by the indicated siRNA reagents targeting 10 different genes (4 siRNAs per gene or a single SMARTpool reagent). Off-targets were quantified using microarray analysis (Agilent) then compiled. Each shaded box represents the middle 50% of the data set. Horizontal line in box: Median value of the data set. Vertical bars: minimum and maximum data values.
- B.D. Parsons, A. Schindler, D.H. Evans, E. Foley, A direct phenotypic comparison of siRNA pools and multiple individual duplexes in a functional assay. PLoS One. 4(12), e8471 (2009).
- M. Jiang, R. Instrell, B. Saunders, H. Berven, M. Howell, Tales from an academic RNAi screening facility; FAQs. Brief Funct. Genomics. 10(4), 227-237. doi: 10.1093/bfgp/elr016 (2011).
- T. Ratovitski, E. Chighladze, E. Waldron, R.R. Hirschhorn, C.A. Ross, Cysteine proteases bleomycin hydrolase and cathepsin Z mediate N-terminal proteolysis and toxicity of mutant huntingtin. J. Biol. Chem. 286(14), 12578-12589 (2011). [Human Proteases]
Product inserts
Related Products
Concentrated buffer solution recommended for resuspension and long-term storage of any short, double-strand, or single-strand synthetic RNA molecule. Dilute with RNase-free water prior to use.
Catalog ID:B-002000-UB-100
Unit Size:100 mL
$99.00
Molecular grade water for dilution of 5x siRNA Buffer or resuspension of RNA. RNase-free to prevent degradation of RNA reagents and oligonucleotides.
Catalog ID:B-003000-WB-100
Unit Size:100 mL
$32.00
An aliquot of each of the four DharmaFECT formulations for siRNA/microRNA transfection optimization studies
Catalog ID:T-2005-01
Unit Size:0.2 mL
