- Gene editing
- Gene editing reagents
- Edit-R HDR Plasmid Donor Primers
Edit-R HDR plasmid donor primers
PCR components of Edit-R Plasmid Donor Kits

Confirmation of proper plasmid assembly is a critical step to ensuring an HDR donor plasmid has been properly assembled and is viable for use as a DNA donor. The Edit-R HDR DNA Donor Plasmid Colony PCR Primer Pairs are essential components of the Edit-R HDR Plasmid Donor Kit and are utilized to confirm that the 5′ and 3′ homology arms are properly inserted in the completed donor template plasmid. Following assembly of the EGFP or mKate2 DNA donor plasmid from the backbone, insert, and two homology arms; a PCR reaction is carried out across the two homology arms from the backbone to the insert region to ensure proper assembly and orientation. To confirm assembly of a donor plasmid containing a custom sequence, a single PCR reaction amplies the entire region encompassing both homology arms and the insert.
Highlights
- Fast and efficient assembly of the DNA donor plasmid
- Validated protocols for design, assembly, and confirmation of the donor plasmid
- Utilize the CRISPR Design Tool for designing the crRNA to the cut site
- Seamless integration with the HDR Donor Designer for designing the primers to create the required 5′ and 3′ homology arms flanking the insertion site
Included Components & Amounts
| Cat # | Description | Item# | Amount |
|---|---|---|---|
| UK-008100-PP-05 | Edit-R HDR DNA Donor Plasmid Colony PCR Primer Pair - EGFP | ||
| Edit-R Colony PCR Primer EGFP Forward | U-008100-FB-05 | 5 nmol | |
| Edit-R Colony PCR Primer EGFP Reverse | U-008100-RA-05 | 5 nmol | |
| UK-008200-PP-05 | Edit-R HDR DNA Donor Plasmid Colony PCR Primer Pair - mKate2 | ||
| Edit-RColony PCR Primer mKate2 Forward | U-008200-FB-05 | 5 nmol | |
| Edit-RColony PCR Primer mKate2 Reverse | U-008200-RA-05 | 5 nmol | |
| UK-008300-PP-05 | Edit-RHDR DNA Donor Plasmid Colony PCR Primer Pair - Universal | ||
| Edit-RColony PCR Primer Backbone Forward | U-008300-FW-05 | 5 nmol | |
| Edit-RColony PCR Primer Backbone Reverse | U-008300-RV-05 | 5 nmol |
Note: The colony PCR primers are included with the HDR Plasmid Donor Kit; they may be ordered separately if more material is needed.
LMNA tagged at the N-terminus with EGFP and SEC61B tagged at the N-terminus with mKate2 in U2OS cells
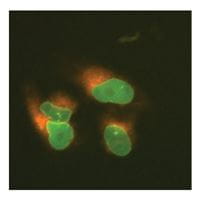
U2OS cells were transfected with DharmaFECT Duo, Cas9 mRNA, synthetic crRNA-tracrRNA targeting LMNA, synthetic crRNA-tracrRNA targeting SEC61B, an EGFP donor plasmid specific to the N-terminus of LMNA containing ~1000 bps of homology per homology arm, and a mKate2 donor plasmid specific to the N-terminus of SEC61B containing ~1000 bps of homology per homology arm. Cells were passaged as needed, and imaged 7-days post transfection on an InCell 2200 high content fluorescent microscope to identify single cells that contained both EGFP and mKate2 insertion.
Detection of successful cloning by colony PCR
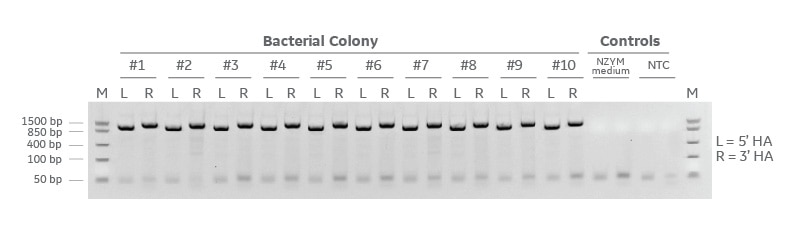
Gibson cloning results of the Edit-R donor plasmid kit with universal backbone, EGFP insert, and two PCR generated homology arms. The resulting cloning reaction was transformed in NEB 5-alpha chemically competent cells and grown at 37 degrees C overnight. 10 bacterial colonies were selected at random and colony PCR was performed to detect the presence of each homology arm (L=5 homology arm and R=3 homology arm). Colony PCR amplicons were run on a 2% agarose E-gel containing ethidium Bromide and a FastRuler low molecular weight molecular weight marker.
Materials required for cloning HDR plasmid donors
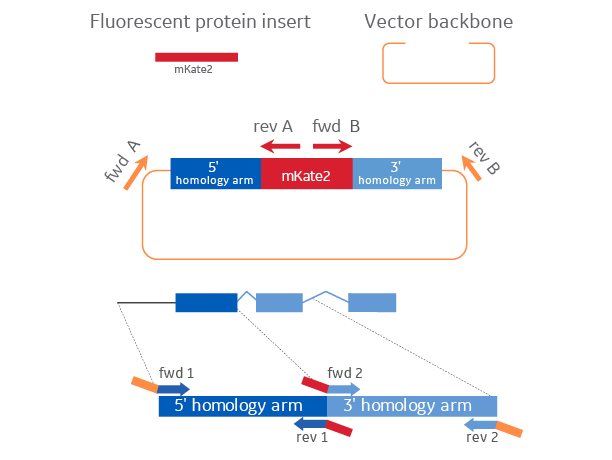
The Edit-R HDR plasmid donor is created through plasmid cloning and assembly of a fluorescent protein insert sequence, the cut vector backbone, and two homology arms generated with custom PCR primers to geneomc regions flanking the insertion site.
Diagram of the plasmid donor cloning workflow
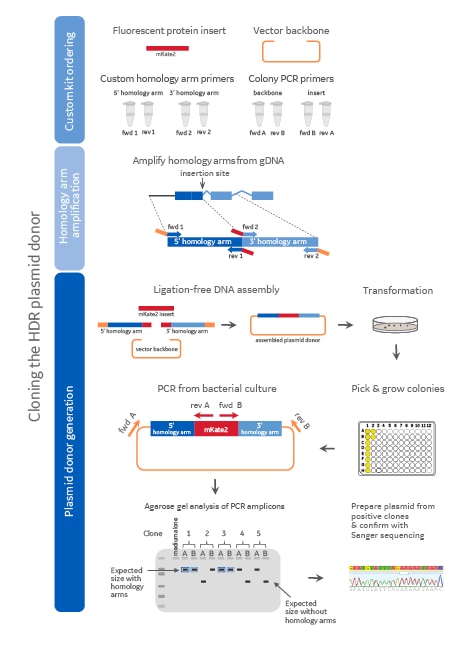
Diagram of the plasmid donor cloning workflow including component ordering, homology arm amplification, and plasmid donor generation. Colors on the diagram indicate the origin of the DNA (dark blue = 5' homology arm, light blue = 3' homology arm, red = mKate2 fluorescent reporter sequence, orange = plasmid backbone). medium alone = media-only negative PCR control.
Workflow to acquire mKate2 knock-in clonal cell line
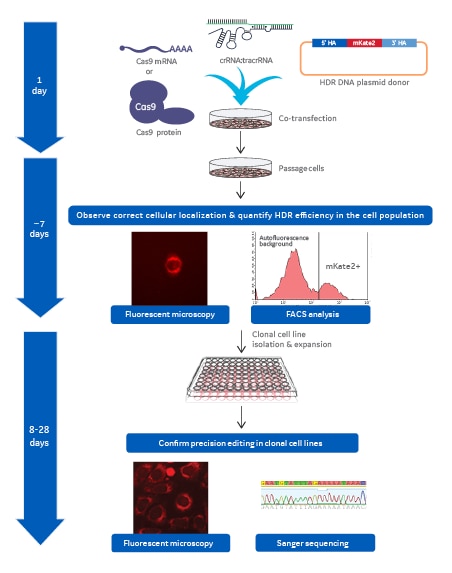
Workflow showing the major process steps and general timing necessary to generate a mKate2 knock-in clonal cell line.
Edit-R mKate2 knock-in donor
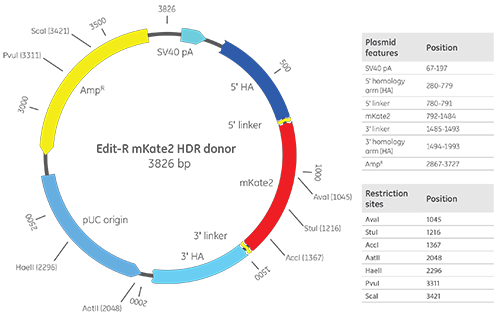
Edit-R mKate2 plasmid donor map with important vector features. The example includes 500 bp homology arms for reference.
Sequence verification of positive clones
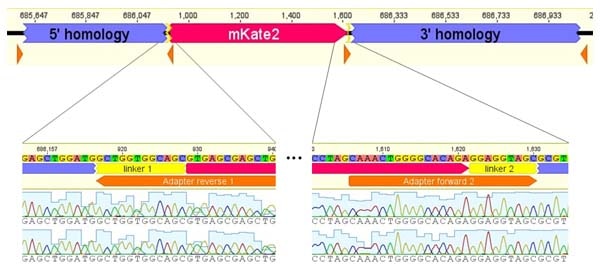
Following colony PCR, Sanger sequencing was utilized to confirm the sequences of positive clones.
Application notes
Product inserts
Safety data sheets
Related Products
Kit for assembly of a plasmid DNA EGFP, mKate2, or custom sequence HDR knock-in donor
