- Dharmacon Screening libraries
- shMIMIC Inducible Lentiviral microRNA Pooled Libraries
shMIMIC Inducible Lentiviral microRNA Pooled Libraries
Take control of microRNA expression for powerful functional screening of hundreds or thousands of microRNAs.


Inducible microRNA expression for highly regulatable functional analysis screens
A novel construct for inducible expression of a mature microRNA, shMIMIC Inducible Lentiviral microRNA pooled libraries are available to target every human and mouse microRNA within in the miRBase database (www.mirbase.org).
Pooled lentiviral libraries offer an efficient and cost-effective method for screening large numbers of microRNAs without automation. shMIMIC pooled libraries are offered for all human and mouse miRBase matures, as well as a Human/Mouse Conserved collection.
Library construction, pooling techniques, and high-throughput sequencing-compatible screening workflows have been experimentally validated to ensure reproducibility and accurate hit identification. Learn about optimal library design and the pooled screening workflow on our Pooled Lentiviral Screening Libraries page.
Highlights:
- Innovative vector design for robust microRNAs robust expression from shMIMIC Inducible Lentiviral microRNAs
- SMARTchoice options let you select the promoter and reporter for optimal performance in your experiment
- Tight regulation of expression from the Tet-On® 3G transactivator protein
- The constructs are designed to preserve the function of the miRNA repression but may deviate slightly from the mirBase sequence
Choose shMIMIC Lentiviral microRNA Pooled Libraries for constitutive expression of mature microRNAs.
Each shMIMIC lentiviral library includes:
- 30 Non-targeting controls
- 80 species-specific positive control shRNAs targeting 10 common viability and reference genes (8 shRNAs each)
- A data file including construct sequences, miRBase gene IDs, and counts per millions of mapped reads
| shMIMIC microRNA Pooled Library | Number of mature microRNAs | Number of unique designs | Number of pools × number of constructs per pool1 |
|---|---|---|---|
| Human | 2580 | 2555 | 1 pool × 2665 constructs |
| Mouse | 1913 | 1896 | 1 pool × 2006 constructs |
| Human-Mouse Conserved | 355 human + 341 mouse | 386 | 1 pool x 496 constructs |
1Total constructs includes 110 non-targeting and gene-specific shRNA constructs. All shMIMIC Lentiviral microRNA pooled libraries are ≥ 5 × 108 TU/mL (+/- 20 %) provided in 8 tubes × 25 µL (200 µL total)
Reagents recommended in our validated protocol:
- Vector-matched SMARTvector Inducible Non-targeting control for transduction optimization
- SMARTvector Indexing PCR and Sequencing Primer Kits (A & B) with 12 unique indexing primers, optimized and experimentally validated for:
- Efficient PCR amplification of genomic DNA with minimal bias
- High-throughput multiplexed Next Gen Sequencing for hit identification
Before ordering, download these valuable tools to help plan your pooled lentiviral screen and calculate the amounts of components required:
- SMARTvector & shMIMIC Lentiviral Pooled Libraries Technical Manual
- Pooled Lentiviral shRNA Screening Laboratory Protocols & Calculation Tracking Worksheet
Important Notice
The shMIMIC Inducible Lentiviral microRNA Pooled Libraries are solely for internal research use (as set forth in the Product Terms and Conditions) in laboratories where the containment measures stated below and in applicable laws and regulations are met. Reagents may not be used for diagnostic, therapeutic or other commercial purposes and may not to be administered to humans for any purpose or to animals for therapeutic purposes. SMARTvector Lentiviral shRNA Pooled Libraries are provided as lentiviral particles are replication-incompetent, self-inactivating (SIN) and non-pathogenic (do not cause infectious human disease).
Any investigator who purchases lentiviral particle products is responsible for consulting with their institution's health and biosafety personnel for specific guidelines on the handling of lentiviral vector particles. Furthermore, each investigator is fully responsible for obtaining the required permissions for research using and the acceptance of replication-incompetent SIN lentiviral vectors and replication-defective lentiviral particles into their local jurisdiction and institution.
Elements of the shMIMIC Inducible microRNA Lentiviral Backbone
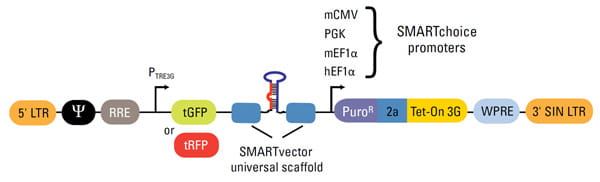
| Vector Element | Utility |
|---|---|
| 5' LTR | 5' Long Terminal Repeat necessary for lentiviral particle production and integration of the construct into the host cell genome |
| Ψ | Psi packaging sequence allows viral genome packaging using lentiviral packaging systems |
| PTRE3G | Inducible promoter with Tetracycline Response Element is activated by the Tet-On® 3G protein in the presence of doxycycline |
| tGFP or tRFP | TurboGFP or TurboRFP reporter for visual tracking expression upon doxycycline induction |
| SMARTvector universal scaffold | Optimized proprietary scaffold based on native primary microRNA in which gene-targeting sequence is embedded |
| PuroR | Puromycin resistance permits antibiotic selection of transduced cells |
| 2a | Self-cleaving peptide enables the expression of both PuroR and Tet-On® 3G transactivator from a single RNA pol II promoter |
| Tet-On® 3G | Encodes the doxycycline-regulated transactivator protein, which binds to PTRE3G promoter only in the presence of doxycycline |
| WPRE | Woodchuck Hepatitis Post-transcriptional Regulatory Element enhances transgene expression in target cells |
| 3' SIN LTR | 3' Self-inactivating Long Terminal Repeat for generation of replication-incompetent lentiviral particles |
An Optimized Inducible Mature microRNA Over-expression System in a Single Vector
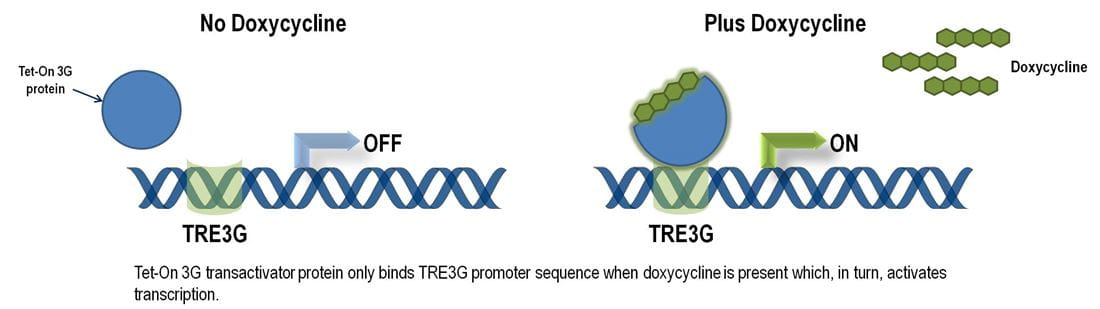
The shMIMIC Inducible microRNA vectors incorporate the Tet-On® 3G bipartite induction system a 3rd-generation Tet-inducible system significantly improved and optimized for minimal basal expression (lowest leakiness) and potent activation upon induction (Zhou X, et al., Gene Therapy 13, 1382 (2006) and Loew R, et al., BMC Biotechnol. 10, 81 (2010)). The Tet-On® 3G Inducible System permits tightly controlled shMIMIC microRNA expression and study of gene function in vivo and in vitro with unprecedented precision.
Pooled shMIMIC Screening Workflow
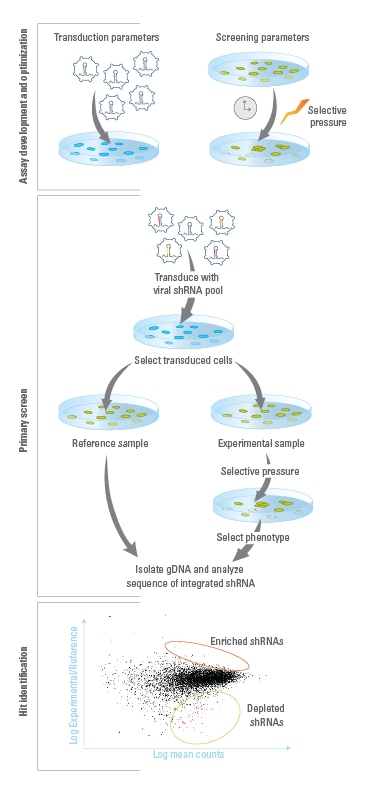
Assay Development and Optimization: Establish optimal experimental conditions, including those for a) lentiviral transduction and b) screening parameters, such as selective pressure and time between collection of reference and experimental samples.Primary Screen: A stable population of cells expressing single integrands of constructs are created by transducing lentiviral pools at low MOIs. Transduced cells are then split into reference and experimental populations for application of a selective pressure that induces the phenotype of interest. Genomic DNA (gDNA) is then isolated from reference and experimental populations of transduced cells. Illumina-adapted primers and Phusion Hot-Start II High Fidelity DNA Polymerase are used to PCR amplify integrated construct sequences and add Illumina flow-cell binding sequences. The resulting amplicons are run on Illumina platform sequencers, using the sequencing primers provided.Hit Identification and Follow-up: Construct sequences are identified in reference and experimental libraries. Constructs that are enriched or depleted during the screen are identified as hits, and the genes that they target are identified. Hits can be confirmed and studied further using individual constructs that can be ordered from the Dharmacon catalog collection.
shMIMIC Inducible microRNA promoter activities differ across cell types.
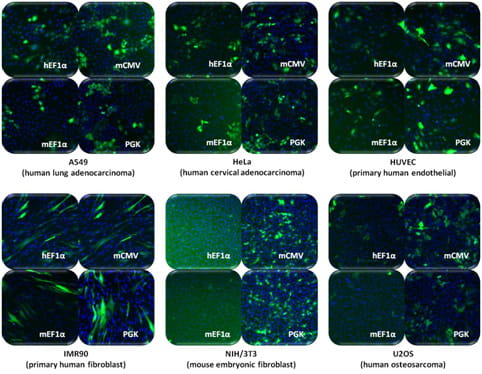
SMARTchoice Inducible Non-targeting Controls were used to transduce the indicated cell types at MOI = 0.3. 24 h after transduction, expression of the non-targeting shRNA and PuroR was induced with 1 µg/mL doxycycline. After 48 h of culture in the presence of doxycycline, cells were stained with Hoescht-33342 and nuclei (blue) and TurboGFP (green) were imaged. While ~30% of the cells in the field have been transduced, some images may appear to contain fewer than 30% TurboGFP-positive cells due to low TurboGFP expression, indicative of low constitutive promoter activity, in a particular cell type.
SMARTvector Inducible Lentiviral shRNAs permit tunable knockdown of specific target genes in human primary cells with all four promoter options.
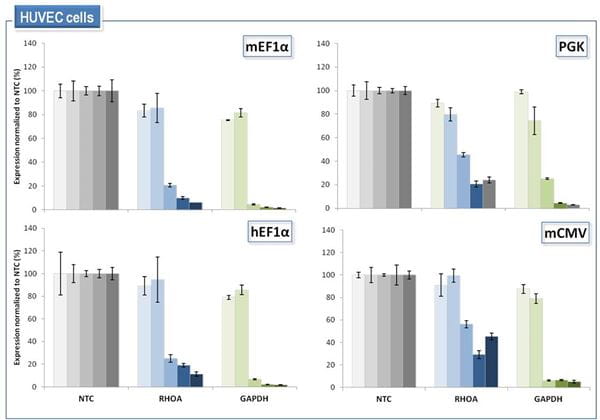
HUVEC (primary human umbilical vein endothelial) cells were transduced at MOI = 0.2 with the indicated SMARTvector Inducible Lentiviral shRNA vectors carrying a non-targeting control shRNA (NTC) or shRNAs directed against RHOA or GAPDH. Cells were selected with 1.5 µg/mL puromycin for 72 hours. shRNA expression was then induced with 0, 0.01, 0.1, 1.0 and 10 µg/mL doxycycline, respectively, and mRNA was harvested 72 hours after dox-induction. Target gene silencing was measured by RT-qPCR relative to the PPIB reference gene. Gene silencing is expressed relative to the vector- and doxycycline dosage-matched NTC cell population.
The Human-Mouse Conserved microRNA Collection
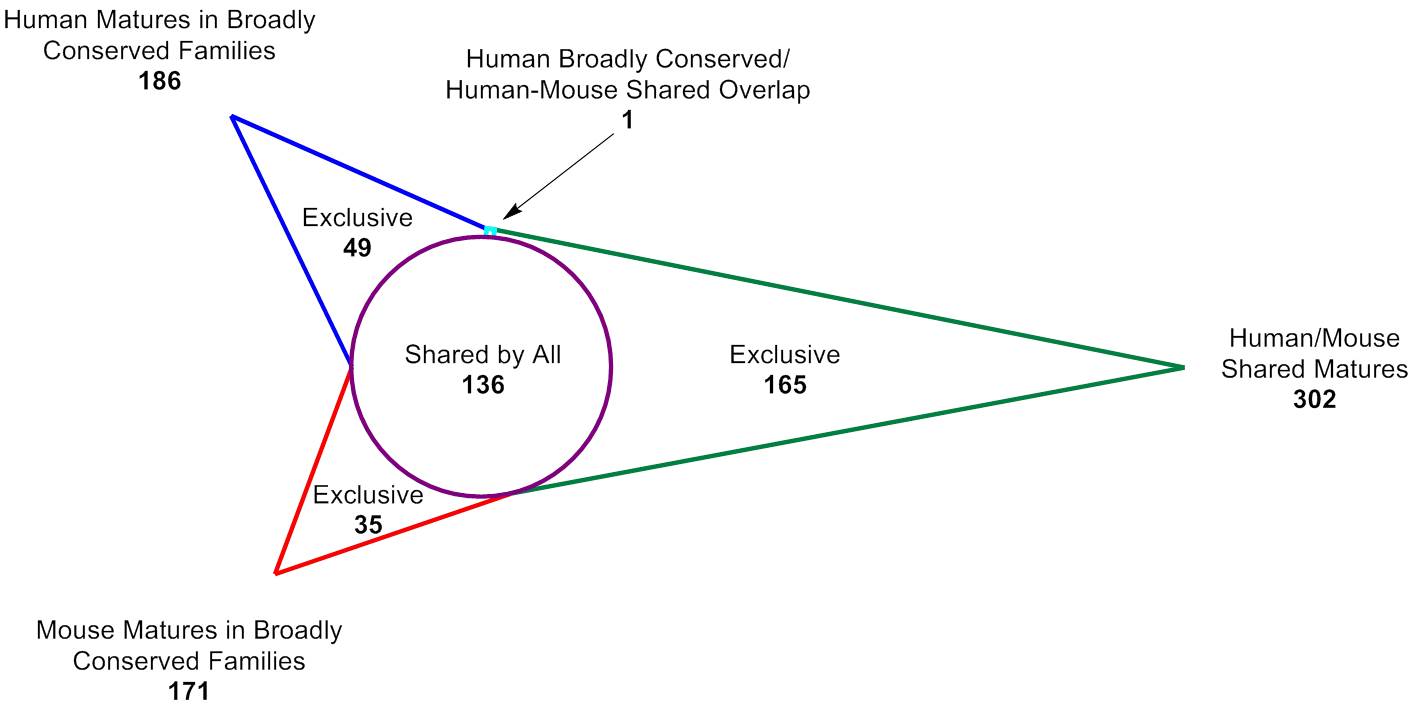
The Human-Mouse Conserved microRNA pooled library is useful for researchers that need ultra-high fold coverage for subtle phenotypes, or who may not have enough cells (e.g. primary cells) for a larger screen and wish to focus on highly conserved microRNAs. It can be used in either human or mouse models, and translates well to in vivo applications.It includes 386 total mature microRNAs:302 human and mouse matures that are identical35 mouse matures in broadly conserved families that are not identical to human49 human matures in broadly conserved families that are not identical to mouseBroadly conserved families as defined at www.targetscan.org
- Tet-inducible expression system references:
- Loewr, Heinz N, et al., Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 10, 81 (2010).
- Zhou X, Vink M, et al., Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 13(19), 1382-1390 (2006).
- Ž. Strezoska, A. Licon, Optimized PCR Conditions and Increased shRNA Fold Representation Improve Reproducibility of Pooled shRNA Screens. PLoS One 7, e42341 (2012).
