- CRISPR activation reagents
- CRISPRmod CRISPRa lentiviral sgRNA
CRISPRmod CRISPRa lentiviral sgRNA
Predesigned CRISPRa lentiviral sgRNAs for highly efficient gene activation in human and mouse models; available as glycerol stocks and high-titer purified particles.

CRISPRmod CRISPRa lentiviral sgRNA
1Start Here
2Choose
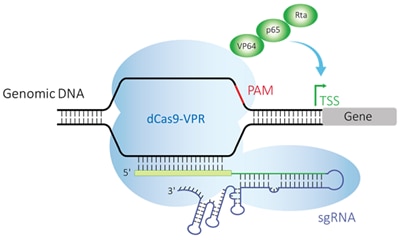
Endogenous gene activation with an easy-to-use lentiviral reagent
The CRISPR activation (CRISPRa) system is a unique adaptation of the classical CRISPR-Cas9 gene editing system which utilizes a catalytically deactivated or dead S. pyogenes Cas9 (dCas9) that is fused to one or more transcriptional activators. When paired with a well-designed guide RNA that targets a gene near a promoter region, or trancriptional start site (TSS), it promotes transcriptional activation.
Review our CRISPRa applications page to get an overview of the technology!
Highlights of CRISPRa Lentiviral sgRNA
- Individual constructs and sets of 4 are available as high-titer purified lentivirus particles or glycerol stocks.
- Designed with a published algorithm by Horlbeck, et. al. that has demonstrated strong levels of gene activation from optimized designs adjacent to one or more transcription start sites.
- Purified, concentrated high-titer lentivirus particles can be directly transduced into cells, including difficult-to-transfect cells and lines.
- Easily select for edited cells with co-expression of puromycin resistance gene.
Required components for an CRISPRa gene activation experiment using lentiviral sgRNA
- A lentiviral expression plasmid or lentiviral particles for CRISPRa dCas9-VPR; a mammalian codon-optimized S. pyogenes deactivated Cas9 fused to VPR activation domains (also compatible with SunTag technology).
- A predesigned CRISPRa lentiviral sgRNA for the gene of interest.
Schematic diagram of the CRISPRa lentiviral sgRNA vector
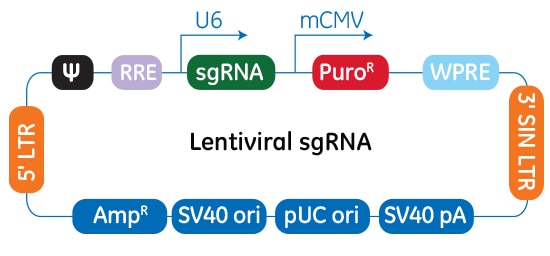
In the CRISPRa lentiviral sgRNA vector backbone, the gene-specific guide RNA is expressed under the control of a human U6 promoter, while expression of the puromycin resistance marker (PuroR) is driven from the mouse CMV promoter and allows for rapid selection of cells with integrated CRISPRa sgRNA. The plasmid contains the AmpR resistance marker for growth and selection in E. coli.
CRISPRa workflow diagram with stable dCas9-VPR expression
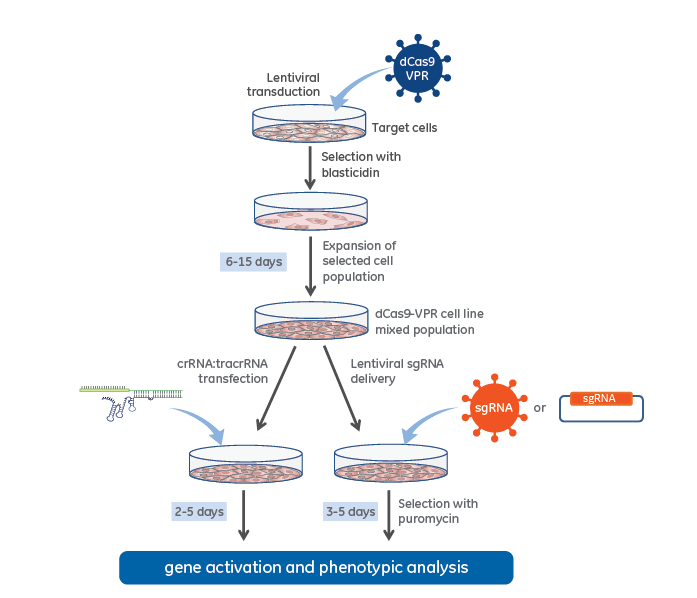
CRISPR activation workflow with lentiviral dCas9-VPR and synthetic crRNA:tracrRNA (left side) or Lentiviral expressed sgRNA (right side).
CRISPRa plasmid co-transfection workflow
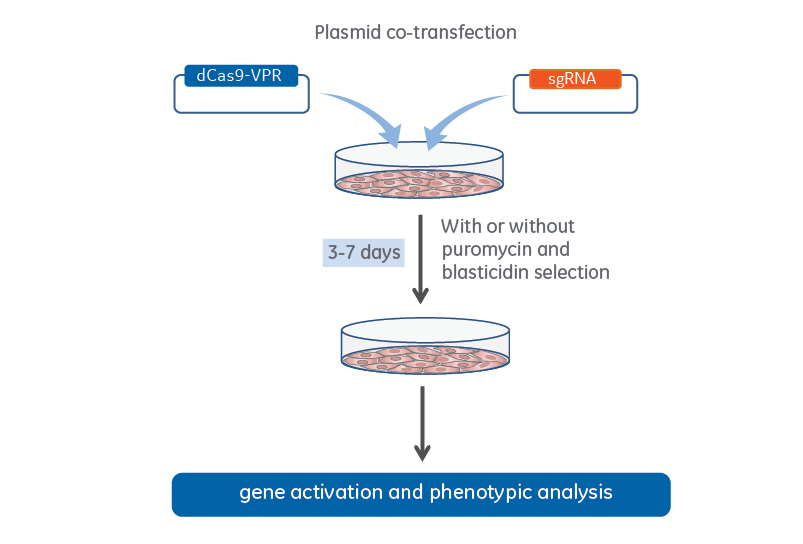
CRISPRa workflow for plasmid co-transfection of lentiviral dCas9-VPR and expressed sgRNA.
CRISPRa lentiviral sgRNA controls
CRISPRa lentiviral sgRNA positive controls
- CRISPRa sgRNAs targeting well-characterized genes to determine the effectiveness of your experimental conditions for maximum activation.
CRISPRa lentiviral sgRNA non-targeting controls
- Non-targeting controls to evaluate baseline cellular responses to CRISPRa components in the absence of target-specific sgRNA.
Related products
CRISPRa dCas9-VPR
- Lentiviral particles, purified plasmid or mRNA that express nuclease-deactivated Cas9 fused to transcriptional activators. When complexed with a guide RNA will trigger an endogenous gene’s expression.
Transductions with CRISPRa lentiviral sgRNA particles in different dCas9-VPR cell lines
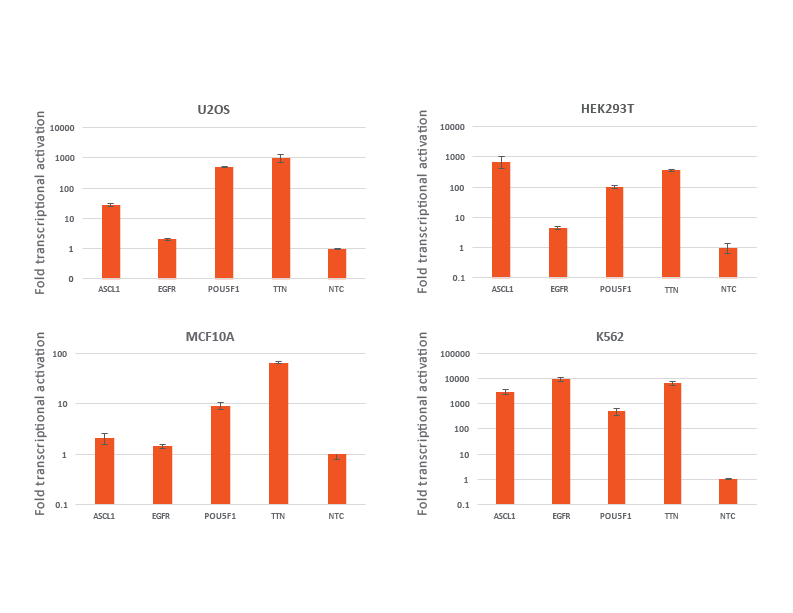
U2OS, HEK293T, MCF 10A and K562 stably expressing integrated CRISPRa dCas9-VPR were plated at 10,000 cells/well and transduced with CRISPRa sgRNA lentiviral particles targeting POU5F1 or TTN at a MOI of 0.3 to obtain cells with a single integrant. Cells were selected with 2 µg/mL puromycin for 4 days prior to analysis with RT-qPCR. The relative expression of each gene was calculated with the Cq method using GAPDH as the housekeeping gene and normalized to a non-targeting control.
CRISPRa in U2OS cells stably expressing integrated dCas9-VPR was compared using different guide RNAs and delivery methods
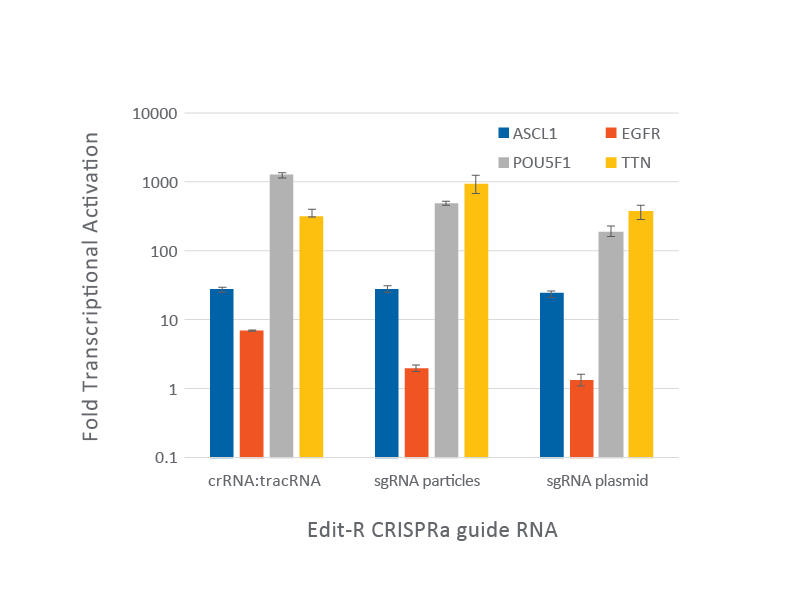
CRISPRa using synthetic crRNA:tracrRNA: Cells were plated at 10,000 cells/well and were transfected using DharmaFECT 4 Transfection Reagent with CRISPRa synthetic crRNA:tracrRNA (25 nM) targeting ASCL1, EGFP, POU5F1 and TTN genes. Cells were harvested 72 hours post-transfection and the relative gene expression was calculated using RT-qPCR. The relative expression of each gene was Cq method using GAPDH as the housekeeping gene and normalized to a non-targeting control.
CRISPRa with lentiviral sgRNA transduction: Cells were plated at 10,000 cells/well and were transduced with CRISPRa sgRNA lentiviral particles targeting ASCL1, EGFR, POU5F1 or TTN at a MOI of 0.3 to obtain cells with a single integrant. Cells were selected with 2 µg/mL puromycin for 4 days prior to analysis with RT-qPCR. The relative expression of each gene was calculated with the Cq method using GAPDH as the housekeeping gene and normalized to a non-targeting control.
CRISPRa with lentiviral sgRNA plasmid transfection: cells were plated at 10,000 cells/well and transfected with CRISPRa sgRNA plasmids (100 ng) targeting ASCL1, EGFR, POU5F1 or TTN using DharmaFECT kb Transfection Reagent. Cells were harvested 72 hours post-transfection (without puromycin selection) and the relative gene expression was calculated using RT-qPCR. The relative expression of each gene was Cq method using GAPDH as the housekeeping gene and normalized to a non-targeting control.
Edit-R lentiviral sgRNA for CRISPRa in human induced pluripotent stem cells
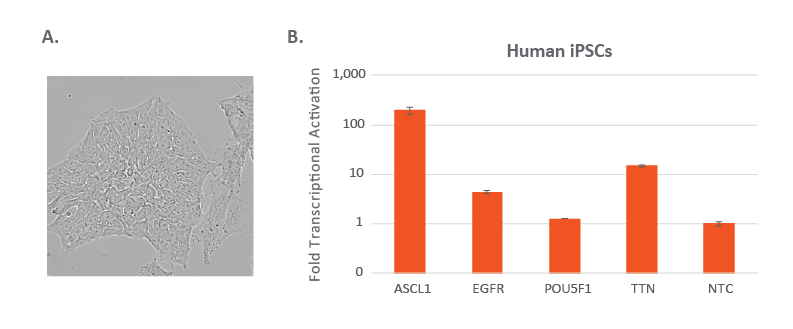
(A) Human iPSC (ThermoFisher Cat #A18945) were cultured in feeder-free and serum free culture conditions and visualized for proper morphology on an inverted fluorescent microscope. (B). The human iPSCs were nucleofected according to manufactures protocols using 96-well shuttle solution P3. Three days after nucleofections, cells were harvested, and prepped for RT-qPCR. The relative expression of each gene was calculated with the Cq method using GAPDH as the housekeeping gene and normalized to a non-targeting control.
- Horlbeck MA, Gilbert LA, et. al., Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. 2016 Sep 23;5. pii: e19760. doi: 10.7554/eLife.19760. PubMed 27661255
-
Chavez A, Scheiman J et. al., Highly efficient Cas9-mediated transcriptional programming Nat Methods. 2015 Mar 2. doi: 10.1038/nmeth.3312. 10.1038/nmeth.3312 PubMed 25730490
-
Tanenbaum ME, Gilbert LA, et. al., A protein tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159(3):635-646. doi:10.1016/j.cell.2014.09.039.
LentiBOOST Lentivirus Transduction Enhancer is a uniquely formulated transduction reagent that can be used with or without lentivirus spinfection in order to increase successful viral transduction events while preserving cell viability. Especially critical for preserving precious primary cells from patient cohorts, or, for engineering complex animal models; improving transduction efficiency can save time and costs by increasing the success of each editing/transduction step, or, even avoid the loss of irreplaceable samples. Additionally, LentiBOOST technology is already used in the manufacturing of a number of clinical stage therapies providing the opportunity to demonstrate improved workflow applicability to the clinic.
LentiBOOST can be purchased through the Dharmacon Reagents catalog.
To learn more about LentiBOOST technology visit the Revvity LentiBOOST webpage.
Supporting Data
Improved CD8+ T-cell SMARTvector™ shRNA lentiviral system transduction using LentiBOOST™ Lentivirus Transduction Enhancer
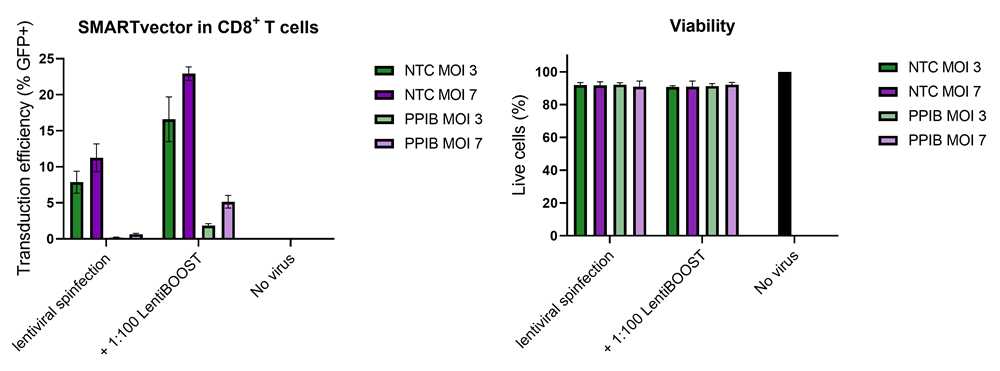
100,000 primary human CD8+ T cells were transduced with either 30,000 (MOI 3, green) or 70,000 (MOI 7, purple) TUs of SMARTVector™ mCMV tGFP Lentiviral Control Particles targeting either NTC or PPIB along with 1:100 LentiBOOST transduction enhancer. Cells were centrifuged at 800 x g for one hour at 32 °C followed by a four hour incubation prior to removal of lentiviral particles and transduction enhancer. Transduction efficiency (%GFP+ out of live cells) and viability were determined 5 days post-transduction by flow cytometry. The addition of LentiBOOST technology markedly improved transduction efficiencies without significantly impacting cell viability.
Improved CD4+ and CD8+ T-cell Edit-R™ All-in-one sgRNA/Cas9 lentiviral system transduction using LentiBOOST™ Lentivirus Transduction Enhancer
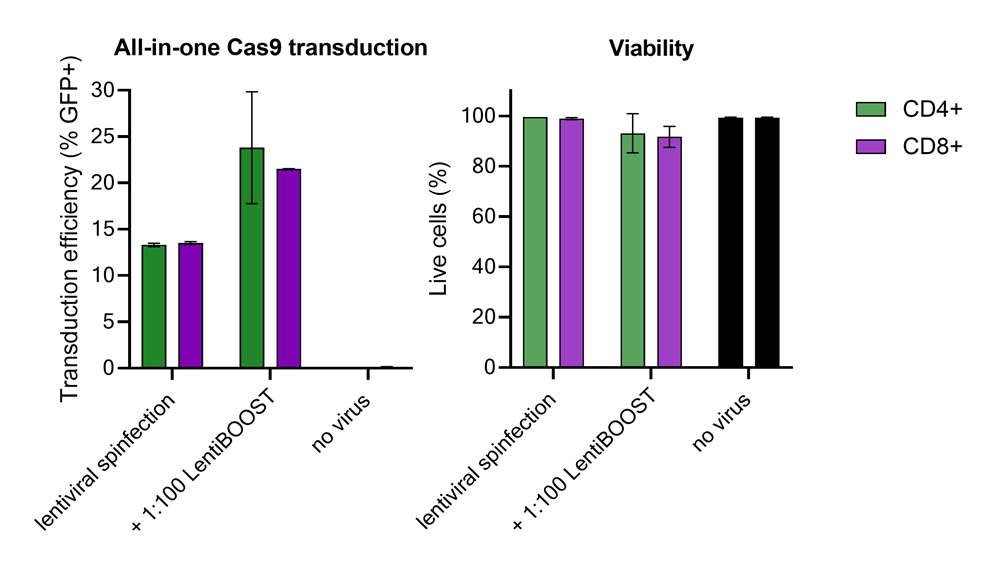
100,000 primary human CD4+ and CD8+ T cells from two donors were transduced with 250,000 TUs of Edit-R GFP Delivery controls mCMV along with 1:100 LentiBOOST transduction enhancer. Cells were centrifuged at 800 x g for one hour at 32 °C followed by an overnight incubation prior to removal of lentiviral particles and transduction enhancer. Transduction efficiency and viability were determined 72 hours post-transduction by flow cytometry. The addition of LentiBOOST technology markedly improved transduction efficiencies without significantly impacting cell viability.
Improved transduction of human induced pluripotent stem cells (hiPSCs) with the Strict-R™ Inducible CRISPRa lentiviral system transduction using LentiBOOST™ Lentivirus Transduction Enhancer

10,000 WTC hiPS cells were transduced with either 20,000 (MOI 2, green) or 40,000 (MOI 4, purple) TUs of Strict-R™ Inducible EGFP dCas9-VPR Lentiviral Particles along with 1:100 LentiBOOST transduction enhancer. Cells were centrifuged at 800 x g for one hour at 32 °C followed by an overnight incubation prior to removal of lentiviral particles and transduction enhancer. Transduction efficiency and viability were determined 72 hours post-transduction by flow cytometry. The addition of LentiBOOST markedly improved transduction efficiencies without significantly impacting cell viability.
Product inserts
Safety data sheets
Related Products
LentiBOOST transduction enhancer can increase successful viral transduction in challenging to transduce cells, or, complex cellular engineering work; while preserving cell viability and minimizing the amount of viral particles required for your experiment. LentiBOOST technology is actively used in the production of clinical stage lentivirally delivered therapies, including some approved therapies, providing a direct path to therapeutic applicability for your research studies. Tested with Dharmacon Lentiviral reagents.
Purified lentiviral particles or plasmid DNA for generation of stable dCas9-VPR nuclease-expressing cell populations
Lentiviral sgRNA constructs bioinformatically designed and validated to not target any gene in human or mouse genomes.
Validated CRISPRa lentiviral sgRNA positive controls for optimization of experimental conditions and quantification of gene activation efficiency.
