Dharmacon™ Edit-R™ reagents are optimized tools for CRISPR-Cas9 gene editing that, when used for genome engineering within a human iPSC line, demonstrate efficient transfection and high rates of indel formation to achieve functional protein knockout.
Here are some of the benefits to creating functional knockout models in human iPSCs using Edit-R Cas9 nuclease mRNA and synthetic sgRNAs.
- Synthetic guide RNA & Cas9 mRNA eliminate the possibility of incorporating unwanted, exogenous DNA into the host cell genome
- Transient expression using synthetic sgRNA and Cas9 nuclease mRNA reduces the risk of off-target editing activity for more specific results
- No incompatibility issues between Cas9 promoter and cell line
- Easy, lipid-based co-transfection of Edit-R synthetic sgRNA and Cas9 mRNA using DharmaFECT Duo Transfection Reagent
Highly efficient transfection with no loss of pluripotency
Following electroporation of Edit-R Cas9 nuclease mRNA co-expressing a GFP reporter, fluorescence was observed in a high percentage of iPSCs just six hours post-transfection, suggesting a highly efficient transfection. Additionally, electroporation of synthetic sgRNAs targeting four genes reported to have no effect on iPSC pluripotency demonstrated >75% indel formation at each locus (TIDE analysis) without impacting pluripotency marker expression.
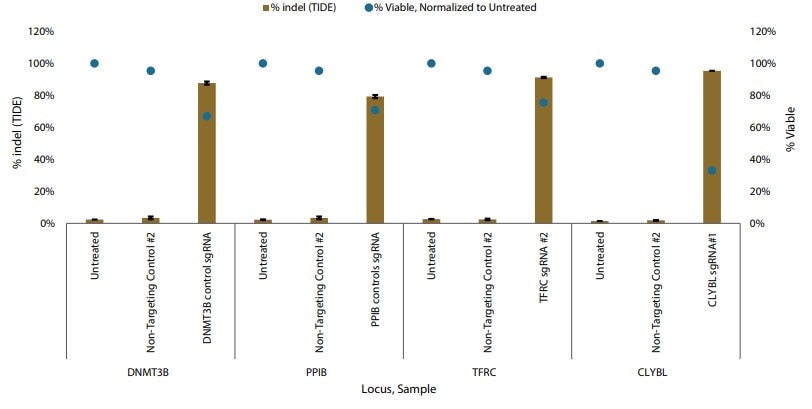
Gene editing in iPSCs with Cas9 nuclease mRNA and synthetic sgRNAs
Figure 1. Gene editing in iPSCs with Cas9 nuclease mRNA and synthetic sgRNAs delivered by nucleofection. Percent indel formation by TIDE analysis.
Indel formation demonstrates a functional knockout phenotype
SOX2 is a known controller of iPSC pluripotency that should induce a loss-of-pluripotency phenotype upon knockout. Electroporation of Edit-R Cas9 nuclease mRNA and three Edit-R synthetic sgRNAs targeting the SOX2 locus caused >80% indel formation for each sgRNA, with a corresponding loss of iPSC morphology. Moreover, staining with antibodies against iPSC pluripotency markers showed diminished expression of SOX2 and loss of OCT4 and TRA-1-60 expression in SOX2 sgRNA-treated samples, while a non-targeting control sample exhibited normal marker expression.
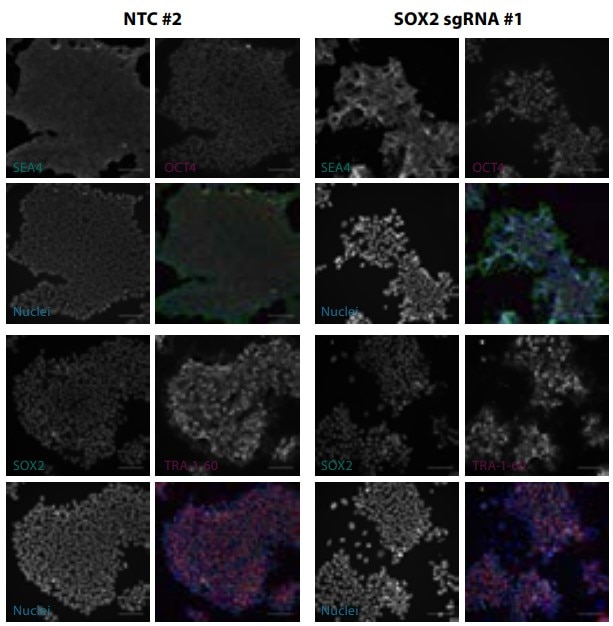
Gene editing in iPSCs with Cas9-mRNA nuclease and synthetic sgRNAs
Figure 2. Gene editing in iPSCs with Cas9-mRNA nuclease and synthetic sgRNAs targeting SOX2. Pluripotency marker staining of fixed cells 5 days after electroporation. Scale bars are 100 μm.
Lipid transfection provides successful delivery of Edit-R gene editing reagents to iPSCs
Although electroporation is the optimal method for delivering Edit-R reagents to iPSCs, it is not always a viable approach. DharmaFECT Duo Transfection Reagent represents an alternative, lipid-based delivery system that provides successful co-transfection of Cas9 mRNA and sgRNAs in iPSCs, as demonstrated by monitoring GFP expression and indel formation.
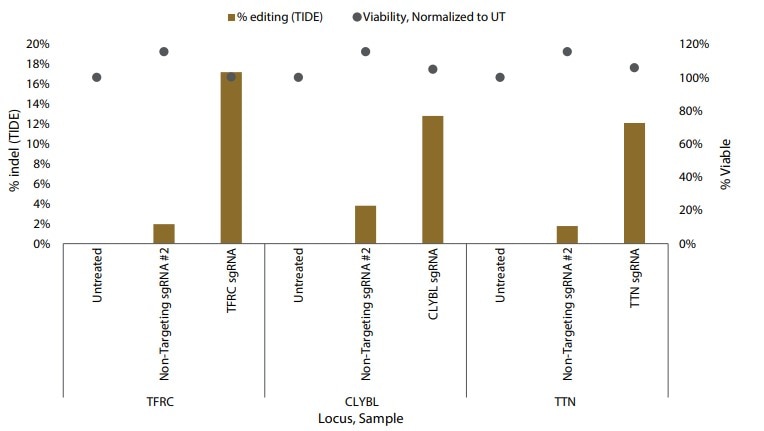
Gene editing in iPSCs with Cas9 nuclease mRNA and synthetic sgRNAs
Figure 3. Gene editing in iPSCs with Cas9 nuclease mRNA and synthetic sgRNAs delivered by DharmaFECT Duo Transfection Reagent. Percent indel formation by TIDE analysis.
To see all the details of generating functional protein knockout in iPSCs using Edit-R reagents, download our application note.
 Written by Ryan Donnelly
Written by Ryan Donnelly
He holds a Professional Science Masters in Molecular Biotechnology from George Washington University and Bachelors in Chemistry from the University of Florida.
Ready to get started with Dharmacon CRISPR-Cas9 gene editing? See our product pages.
Gene editing reagents and cell models
To learn more about the applications of CRISPR-Cas9 gene editing. Check out our applications pages.
Do you have further questions or need some help troubleshooting your experiment, contact our Scientific Support team anytime.
