Precision medicine is defined by the Precision Medicine Initiative as, "an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person". It allows doctors to prescribe treatment tailored to the individual patient and allows drug developers to create treatments that are more targeted to those individuals.
In summary, precision medicine aims to find the right drug, for the right patient, at the right time.
Targeted therapy not only benefits the patient, but also has a positive effect on healthcare costs and success:
- Increased targeted therapy
- Increased clinical trial selection
- Decreased non-targeted selection
- Increased quality of hospice care
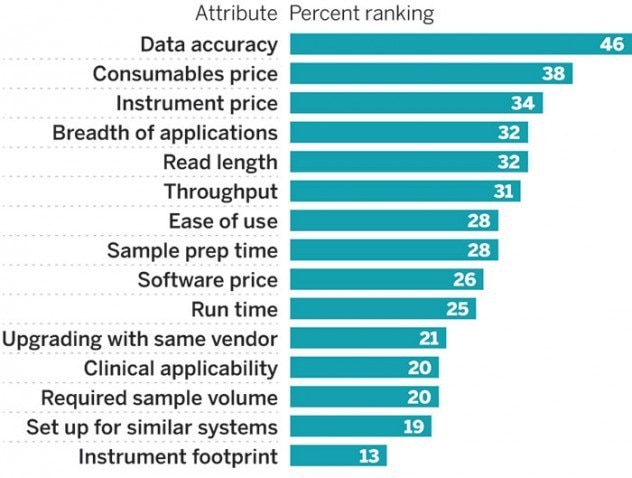
Precision medicine requires accuracy
To enable the ultimate goal of precision medicine, diagnosis needs to be as accurate as possible.
Accurate diagnostics allows you to take an unknown tumor and genotype it, resulting in the most suitable prescription of treatment. This also aids disease monitoring of development of resistance and treatment success.
Accuracy is commonly identified as one of the most important parameters clinical labs are concerned with.
The implications of inaccurate results
We are all aware of the importance of accuracy in our processes and workflows, but what happens if we aren't accurate?
There are a lot of stakeholders involved.
The impact on the patient
False positive results
Patients could undergo unnecessary treatments and potentially a delayed diagnosis of their true condition. Such false positives can be even more detrimental when the test is for ovarian cancer, which could result in ovary removal a significant life change.
False negative results
A test may suggest that a patient doesn't have a disease or condition, when in fact they do. That's the case for a test for the gene mutation that makes an excess of human epidermal growth factor receptor 2 (HER2), which promotes the growth of breast cancer cells. Patients who express HER2 typically take drugs that target HER2, in addition to standard chemotherapy. Most tests used to detect HER2 protein or gene amplification are laboratory-developed tests but in the past, approximately 20% of these tests may have been inaccurate. That means that some breast cancer patients may not receive the best treatment when the test fails to detect high HER2 levels.
The impact on laboratories
In any business, time is a valuable resource and it costs! This doesn't include all the other running costs of a lab:
- Assay
- Equipment
- Consumables
- Staff
- Validation maintenance
- Assay design or change
- And the list goes on
An even greater cost to business is reputational what if the inaccuracy reaches the news?
What are the solutions?
Reference Standards are a key player in increasing and confirming the accuracy of diagnostic testing.
Reference material (Standards) is defined as, "material or substance, one or more of whose property values are sufficiently homogeneous and well established to be used for the calibration of a measuring system, the assessment of a measurement procedure, or for assigning values to materials." (International Organization for Standardization [ISO] 15195).
There are three main types of reference standards:
- Cell-line derived reference standards - these more accurately reflect patient samples than synthetics as they are produced from human cell lines, giving you more accurate quantitative results.
- Synthetic Reference Standards - these are "renewable" as they are provided in oligonucleotides or plasmids. However, due to their nature, they tend to amplify more readily, which can skew any quantitative data.
- The ideal "Gold Standard" would be to use patient-derived reference standards (DIY), but currently these come with a high degree of variability and complexity that makes consistency very difficult to achieve
Laboratories face the challenge of sourcing reference materials that truly reflect patient samples with a defined copy number, precise allele burden and mimics genome complexity and tumor cell genetics, but are also renewable, consistent and reproducible.
Redefining standards with cell line-derived material
The advantages of using cell line-derived Reference Standards:
- Mimics individual patient genetics
- Variants presented in relevant genomic context
- Access to rare mutations and range of allelic frequencies>
- Quality controlled and validated
- Prepared under a certified quality management system
- Renewable and affordable
Horizon uses highly characterized cell lines to develop reference standards. This gives us highly defined material to create reference standards which mimic patient material. Better still, these Reference Standards are consistent, renewable and reproducible.
These offer the option of generating highly customized materials which mimic clinical archives and disease states. Because the variants are engineered directly into human cell lines, they are presented in the appropriate genomic context with all the complexity and diversity that is included in a true genomic sample.
This approach allows us to access specific parameters that are important to evaluate for, including variant type, variant size, and both local and global sequence context. With engineered cell lines, large structural variants and copy number variants in true genomic context can be realized. Using mixtures of mutant and wild type cell lines, we can provide materials at a broad range of allelic frequencies, tailored to the appropriate assay or disease state. This also provides an easy means for determining the limit of detection of an assay, an essential step in assay development
Finally, the ability to multiplex desired variants into single samples means Horizon's Reference Standards are more affordable for routine use.
We're only at the beginning of precision medicine
We are only at the beginning of precision medicine - when applied correctly, personalized medicine can help identify patients who are most likely to benefit from a particular therapeutic product, as well those likely to be at increased risk of relapse or side-effects. We're excited to see what happens next.
