- CRISPR interference reagents
- Strict-R Inducible CRISPRi Lentiviral System
Strict-R Inducible CRISPRi Lentiviral System
Unlock precise, tunable gene repression with the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System. This first-of-its-kind tool enables stringent, reversible gene silencing with exceptional specificity and minimal background activity. Powered by the synergistic combination of the Tet-On 3G and FKBP12-derived degron systems, this platform allows inducible repression and stabilization of dCas9-SALL1-SDS3 in response to small-molecule regulators, providing precise temporal control over target gene repression.
Dual-inducible control for precise gene repression – how it works
System OFF – tight regulation without inducers:
In the absence of doxycycline and Shield1, the system remains inactive. Any minimal transcriptional leakiness from the TRE3G promoter produces a degron-tagged dCas9-SALL1-SDS3, which undergoes rapid proteosomal degradation, ensuring minimal background activity.
System ON – robust, controlled gene silencing:
When doxycycline is added, strong transcription occurs from the TRE3G promoter. Concurrent addition of Shield1 stabilizes the degron-tagged dCas9-SALL1-SDS3 protein, enabling powerful and precise target gene repression in the presence of a specific sgRNA.
Highlights
- Achieve reversible, small molecule-induced repression of target genes with tight, tunable control of gene expression.
- The dCas9-SALL1-SDS3 fusion protein delivers potent transcriptional silencing with minimal off-target activity.
- Integration of the Tet-Degron system enables induction and stabilization of dCas9-SALL1-SDS3 upon addition of doxycycline and Shield1, allowing precise, time-controlled regulation of gene repression.
- The system is delivered through a single lentiviral vector, ensuring seamless integration into existing experimental workflows.
- Ideal for gene expression studies, functional genomics, and screening applications, this platform offers high-quality, purified lentiviral particles for direct transduction with minimal cytotoxicity (≥1 × 10⁷ TU/mL).
- Available with Hygromycin selection or EGFP fluorescent reporter options for versatile experimental design.
Schematic map of the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral vectors
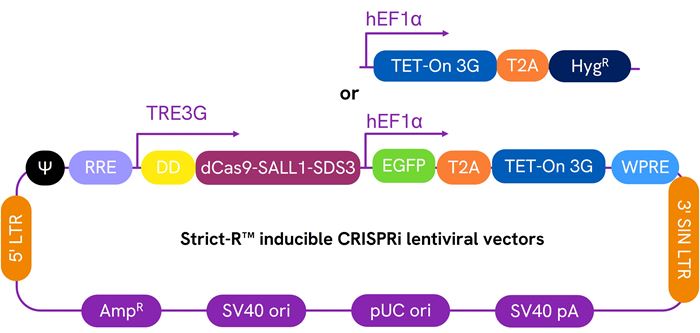
Transcriptional and post-translational control with the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System
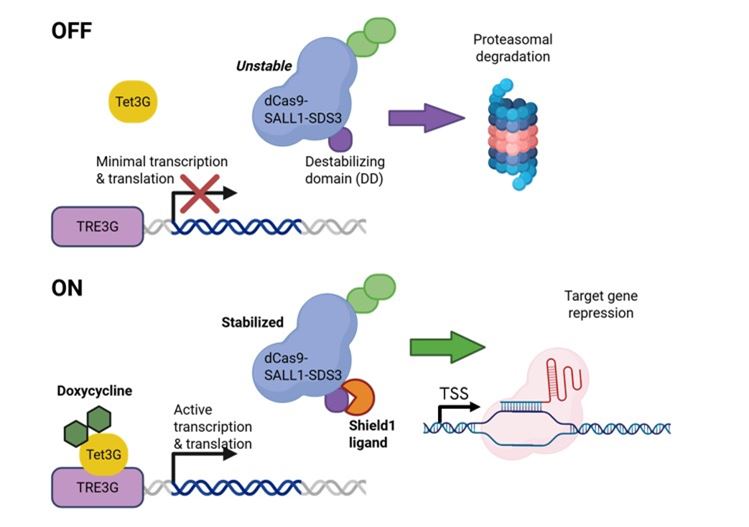
Diagram of the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System. In the absence of doxycycline and Shield1, the system is “OFF”. Leaky bursts of transcription from the TRE3G promoter result in the translation of dCas9-SALL1-SDS3 fused to a FKBP12-derived destabilizing domain (degron) that tags the protein for rapid proteasomal degradation. The addition of doxycycline induces potent transcription from the TRE3G promoter and the addition of Shield1 stabilizes dCas9-SALL1-SDS3 thereby enabling robust target gene repression in the presence of a gene-specific sgRNA. Diagram created with BioRender.com.
Gene repression workflow using the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System
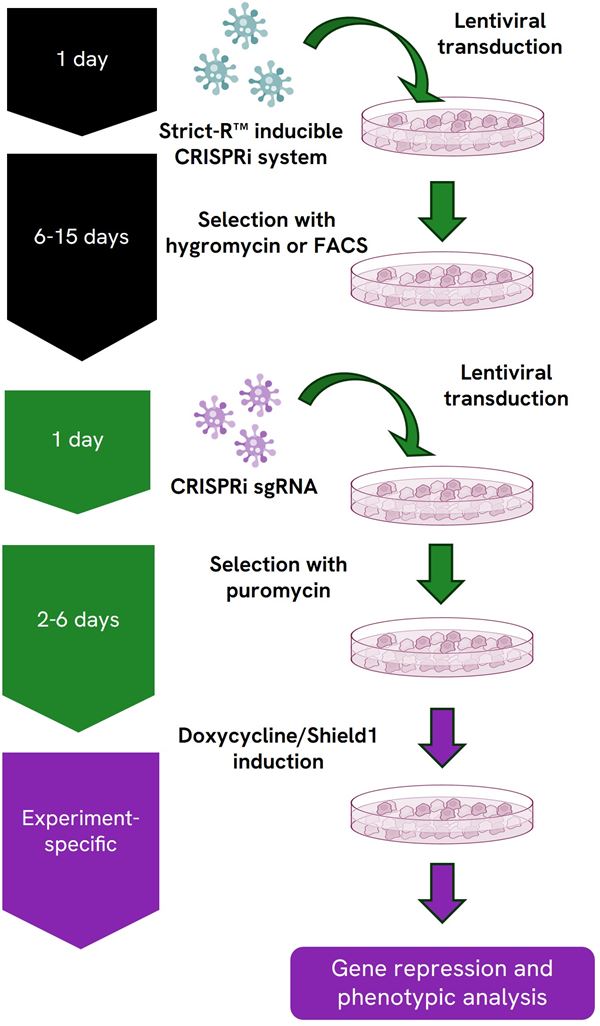
Gene repression workflow using the Strict-R Inducible CRISPRi Lentiviral System
Robust transcriptional repression and minimal leakiness with the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System across cell lines
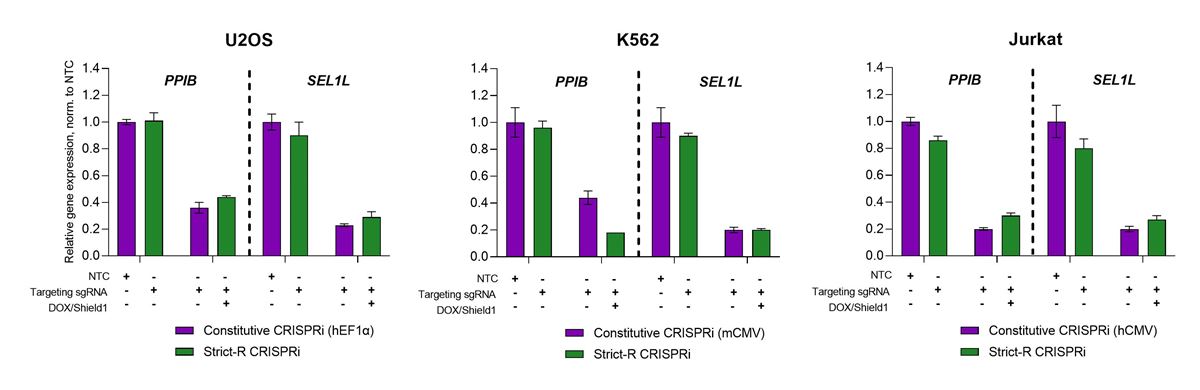
U2OS, K562, and Jurkat cells expressing either the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System (green) or dCas9-SALL1-SDS3 under control of the listed constitutive promoter (purple; hEF1α, mCMV, or hCMV, selected for optimal activity in the respective cell line) were transduced with CRISPRmod CRISPRi lentiviral sgRNA targeting PPIB or SEL1L (labeled as targeting sgRNA +) or a CRISPRmod CRISPRi lentiviral non-targeting control (labeled as NTC +) at MOIs of 0.3. After 48 hours, cells were selected with 2.5 µg/µL puromycin for 5 to 7 days. Cells were plated in 96-well plates at 20,000 cells/well and stimulated with 0.5 µg/ mL doxycycline and 500 nM Shield1 for 48 hours prior to harvest. Total RNA was isolated and relative gene expression was measured using RT-qPCR. The relative expression of each gene was calculated with the ∆∆Cq method using ACTB as the housekeeping gene and normalized to an NTC.
Potent, reversible gene repression with the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System in K562 cells
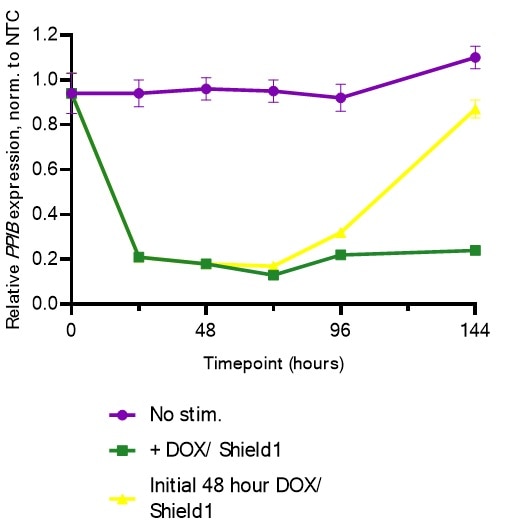
K562 cells expressing the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System were transduced with CRISPRi lentiviral sgRNA targeting PPIB or a CRISPRi lentiviral non-targeting control (NTC) at MOIs of 0.3. After 48 hours, cells were selected with 2.5 µg/µL puromycin for 5 days. Cells were plated in 6-well plates at 200,000 cells/well and stimulated with 0.5 µg/ mL doxycycline and 500 nM Shield1 with small molecule-containing media replenished every 48 hours. Populations only receiving an initial pulse were washed with PBS after 48 hours and subsequently cultured in media without the listed small molecules for the remainder of the experiment. At each time point, aliquots of cells were harvested, total RNA was isolated, and relative gene expression was measured using RT-qPCR. The relative expression of PPIB was calculated with the ∆∆Cq method using ACTB as the housekeeping gene and normalized to an NTC.
Raising Shield1 concentrations up to 500 nM result in more robust gene repression with the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System

U2OS cells expressing the Dharmacon™ Strict-R™ Inducible CRISPRi Lentiviral System were transduced with CRISPRi lentiviral sgRNA targeting PPIB or SEL1L, or a CRISPRi lentiviral non-targeting control (NTC) at MOIs of 0.3. After 48 hours, cells were selected with 2.5 µg/µL puromycin for 5 days. Cells were plated in 96-well plates at 10,000 cells/well and stimulated with media containing 0.5 µg/ mL doxycycline and the listed concentrations of Shield1. After 48 hours, cells were harvested, total RNA was isolated, and relative gene expression was measured using RT-qPCR. The relative expression of each gene was calculated with the ∆∆Cq method using ACTB as the housekeeping gene and normalized to an NTC.
- Bhaya, et al., CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 45, 273-297 (2011).
- M. Jinek, et al., A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337, 816-821 (2012).
- L.S. Qi, et al., Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 152, 1173-83 (2013).
- L.A.Gilbert, et al., CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 154, 442-51 (2013).
- A.W. Cheng, et al., Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res., 23, 1163-71 (2013).
- L. A. Gilbert, et al., Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 159, 647–661 (2014).
- M. E. Tanenbaum, et al., A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 159, 635–646 (2014).
- S. Konermann, et al., Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 517, 583–588 (2015).
- R. Loew, et al., Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 10, 81 (2010).
- Banaszynski, Laura A et al., A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 126, 995-1004 (2006).
- Egeler, Emily L et al., Ligand-switchable substrates for a ubiquitin-proteasome system. The Journal of Biological Chemistry. 286, 31328-36 (2011).
- M. A. Horlbeck et al., Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. eLife. 5, e19760 (2016).
- Maynard-Smith, Lystranne A et al., A directed approach for engineering conditional protein stability using biologically silent small molecules. The Journal of Biological Chemistry. 282, 24866-72 (2007).
- Clarence Mills et al., A Novel CRISPR Interference Effector Enabling Functional Gene Characterization with Synthetic Guide RNAs. CRISPR J 2022 Dec;5(6):769-786.
LentiBOOST Lentivirus Transduction Enhancer is a uniquely formulated transduction reagent that can be used with or without lentivirus spinfection in order to increase successful viral transduction events while preserving cell viability. Especially critical for preserving precious primary cells from patient cohorts, or, for engineering complex animal models; improving transduction efficiency can save time and costs by increasing the success of each editing/transduction step, or, even avoid the loss of irreplaceable samples. Additionally, LentiBOOST technology is already used in the manufacturing of a number of clinical stage therapies providing the opportunity to demonstrate improved workflow applicability to the clinic.
LentiBOOST can be purchased through the Dharmacon Reagents catalog.
To learn more about LentiBOOST technology visit the Revvity LentiBOOST webpage.
Supporting Data
Improved CD8+ T-cell SMARTvector™ shRNA lentiviral system transduction using LentiBOOST™ Lentivirus Transduction Enhancer
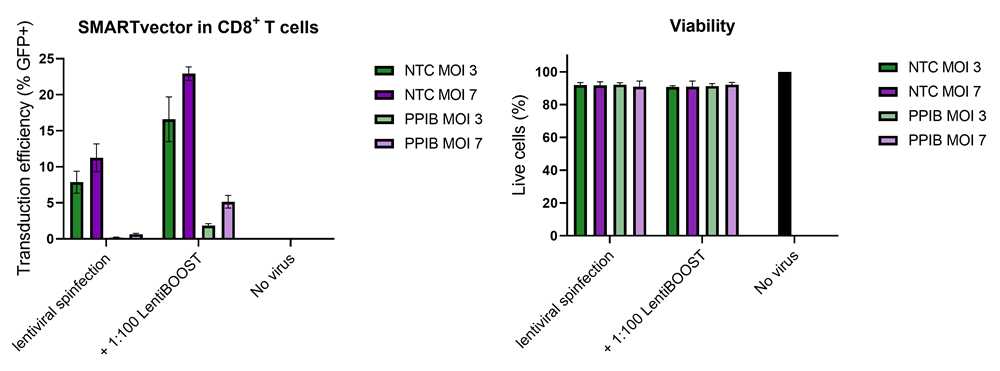
100,000 primary human CD8+ T cells were transduced with either 30,000 (MOI 3, green) or 70,000 (MOI 7, purple) TUs of SMARTVector™ mCMV tGFP Lentiviral Control Particles targeting either NTC or PPIB along with 1:100 LentiBOOST transduction enhancer. Cells were centrifuged at 800 x g for one hour at 32 °C followed by a four hour incubation prior to removal of lentiviral particles and transduction enhancer. Transduction efficiency (%GFP+ out of live cells) and viability were determined 5 days post-transduction by flow cytometry. The addition of LentiBOOST technology markedly improved transduction efficiencies without significantly impacting cell viability.
Improved CD4+ and CD8+ T-cell Edit-R™ All-in-one sgRNA/Cas9 lentiviral system transduction using LentiBOOST™ Lentivirus Transduction Enhancer
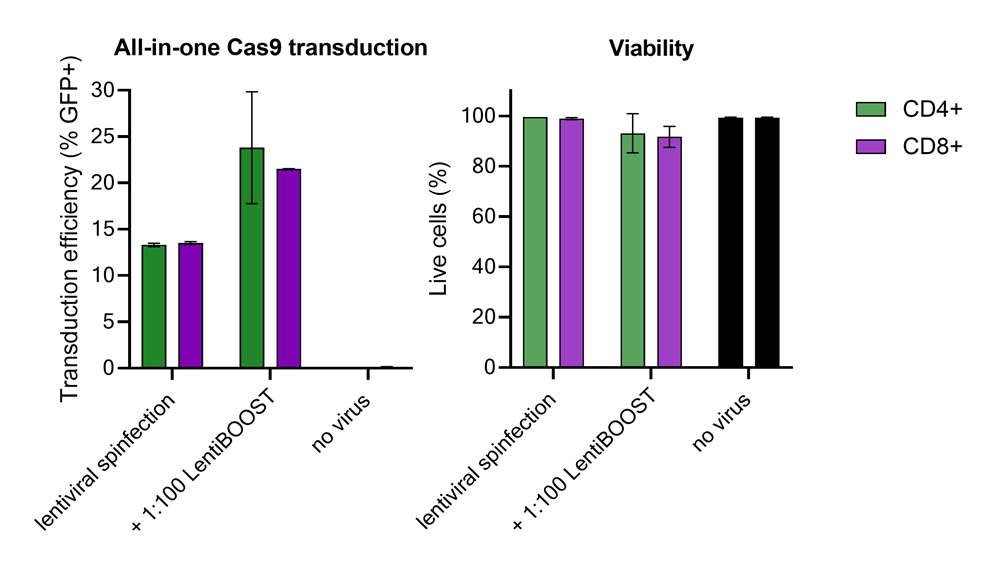
100,000 primary human CD4+ and CD8+ T cells from two donors were transduced with 250,000 TUs of Edit-R GFP Delivery controls mCMV along with 1:100 LentiBOOST transduction enhancer. Cells were centrifuged at 800 x g for one hour at 32 °C followed by an overnight incubation prior to removal of lentiviral particles and transduction enhancer. Transduction efficiency and viability were determined 72 hours post-transduction by flow cytometry. The addition of LentiBOOST technology markedly improved transduction efficiencies without significantly impacting cell viability.
Improved transduction of human induced pluripotent stem cells (hiPSCs) with the Strict-R™ Inducible CRISPRa lentiviral system transduction using LentiBOOST™ Lentivirus Transduction Enhancer

10,000 WTC hiPS cells were transduced with either 20,000 (MOI 2, green) or 40,000 (MOI 4, purple) TUs of Strict-R™ Inducible EGFP dCas9-VPR Lentiviral Particles along with 1:100 LentiBOOST transduction enhancer. Cells were centrifuged at 800 x g for one hour at 32 °C followed by an overnight incubation prior to removal of lentiviral particles and transduction enhancer. Transduction efficiency and viability were determined 72 hours post-transduction by flow cytometry. The addition of LentiBOOST markedly improved transduction efficiencies without significantly impacting cell viability.
Posters
Safety data sheets
Related Products
LentiBOOST transduction enhancer can increase successful viral transduction in challenging to transduce cells, or, complex cellular engineering work; while preserving cell viability and minimizing the amount of viral particles required for your experiment. LentiBOOST technology is actively used in the production of clinical stage lentivirally delivered therapies, including some approved therapies, providing a direct path to therapeutic applicability for your research studies. Tested with Dharmacon Lentiviral reagents.
Predesigned CRISPRi lentiviral sgRNAs for highly efficient gene repression of any human gene.
Predesigned CRISPRi synthetic sgRNA for highly efficient gene repression of any human protein-coding gene
Validated CRISPRi lentiviral sgRNA for optimization of transcriptional repression experiments.
