Following transfection of CRISPR reagents, cells will need to be single cell diluted to obtain a clonal population. There are several ways of doing this. Read our protocol where we detail the single cell dilution protocol used at Horizon.
If a cell line tolerates being single cell diluted then plating 96-well plates at 1 cell per well in standard cell culture media is appropriate.
If the cell line does not tolerate single cell dilution in standard media, then the use of conditioned media can often improve clone recovery. If the use of conditioned media in 96 well plates does not improve the recovery of clones, cells can be plated to large tissue culture dishes and individual colonies picked.
Single cell dilution protocol for transfected (adherent) cells in 96-well plates
Reagents and equipment required:
- 15 mL and 50 mL conical bottom tubes
- 20 µL and 200 µL filter pipette tips
- Single channel manual pipettes or similar
- 1.5 mL microcentrifuge tubes
- 5, 10 and 25 mL stripettes
- 10 mL sterile reagent reservoir
- multichannel pipettes or similar
- 96 well cell culture plates (up to 30)
- Cell culture media
- Phosphate buffered saline (PBS)
- TrypLE™ (Life Technologies 12605010) cell dissociation reagent or similar
- 250 mL sterile bottles
- Disposable hemocytometer (or equivalent)
- Trypan blue
- Class II Microbiological Safety Cabinet (MSC)
Day 1: Single cell dilution
- Warm media for at least 10 minutes at 37ºC in a water bath.
- Transfer cell culture vessel containing transfected cells from the humidified CO2 incubator to a MSC.
- Remove existing media from cells and dispose to waste bottle.
- Wash cells with PBS.
- Aspirate PBS from flask and place in waste bottle.
- Add 1x TrypLE™ (or other appropriate cell dissociation reagent) to cells (adjust volumes of TrypLE™ added according to the size of the cell culture vessel).
- Incubate the cells for 5 minutes at 37ºC and 5% CO2 until the cells detach.
- Transfer flask back to MSC.
- Triturate cells 3-4 times with 5ml stripette to break up any cell clumps
- Add equal volume of media to that of the TrypLE™ added to the flask to neutralize the TrypLE™ activity.
- Transfer the cells to a 15 mL conical bottom tube.
- Add 20 µL 1x Trypan Blue to a microcentrifuge tube.
- Add 20 µL neutralized cells (from step 11) to the same microcentrifuge tube.
- Mix cells by gentle pipetting.
- Transfer 10 µL of cell suspension to a well of disposable hemocytometer.
- Transfer the hemocytometer to an inverted microscope.
- Count the number of live cells in 3 fields of the hemocytometer (where each field is a 4x4 grid) and determine the mean of these counts [(count1+ count2 + count3)/3]
- Calculate the concentration of cells ( cells/mL) in the cell suspension: Concentration (cells/ml) = Mean x 2 x 10 4
- Dilute cells to 1 x 104 cells/mL in 1 mL of fresh media: volume of cells to add (in ml) can be calculated as follows:
- (1x104) x volume required (mL) / Cell concentration (from step 18)
- Below is the calculated volume of cells (at 1x104 cells per mL) required to be added to 220 mL media for ten 96-well plates for each of 3 cell densities:
1 cell/well (5 cells/mL) 2 cells/well (10 cells/mL) 5 cells/well (25 cells/mL) 5 (cells/mL) x 220 (mL) / 1x104 (cells/mL) 10 (cells/mL) x 220 (mL) / 1x104 (cells/mL) 25 (cells/mL) x 220 (mL) / 1x104 (cells/mL) 110 µL 220 µL 550 µL - Aliquot 220 mL of complete cell culture media into a suitable sterile vessel (e.g. 250 mL sterile bottle). Label each vessel appropriately with the cell concentration.
- To the media in the bottle add the appropriate volume of cell suspension:
- Transfer the diluted cells (1 cell/well, 2 cells/well, 5 cells/well) to large reservoir.
- Dispense 200 µL/well in all 12 columns of ten 96-well plates using a multichannel pipette.
- Dispose of excess cell suspension to waste bottle.
- Repeat steps 23 to 25 for each cell dilution
- Dispose of waste according to the local regulations.
- Transfer plates to a humidified incubator at 37ºC, 5% CO2.
- Visually inspect the plates periodically from 7 to 10 days under a microscope to assess if colonies are establishing.
Day 10-14: Visual identification
30. Visually screen plates using a microscope for clear single colonies. Mark these by circling the well on the lid and/or record in a spreadsheet. When colonies reach 70% confluence, the cells may be harvested to assess for the engineering event.
Example images of single cell dilution
Below are some examples taken using a Cell Metric of both an adherent (HCT116) and suspension (Nalm6) cell lines.
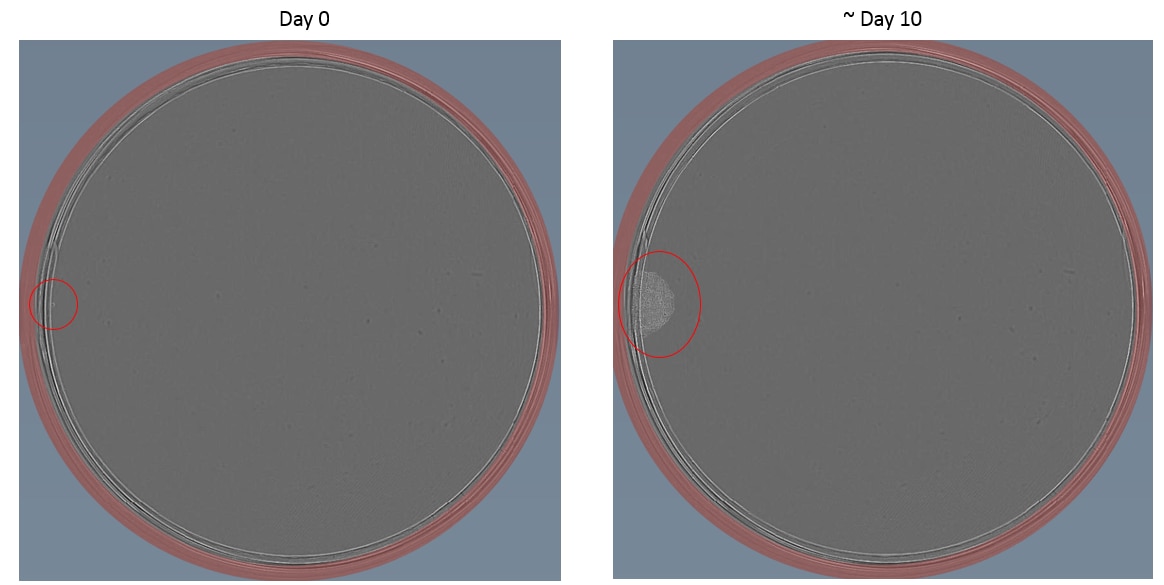
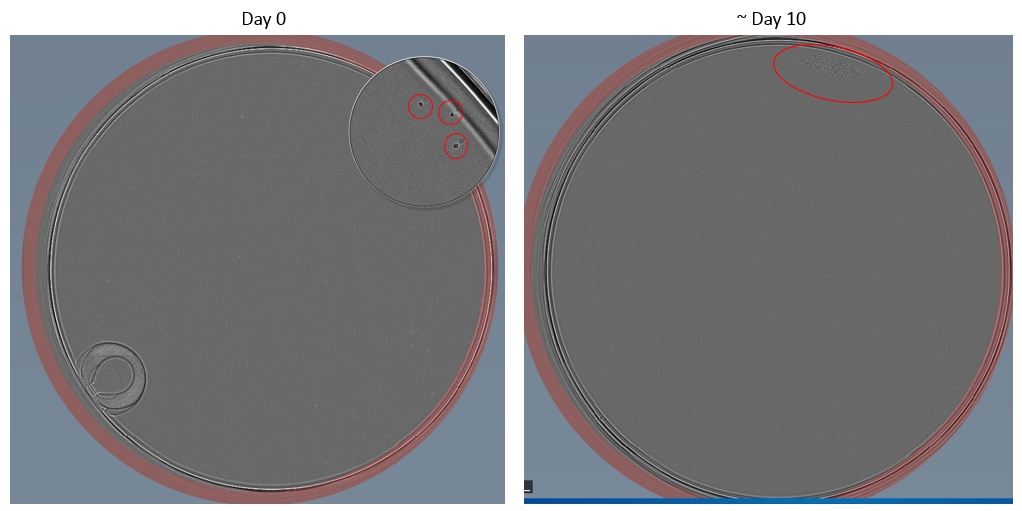
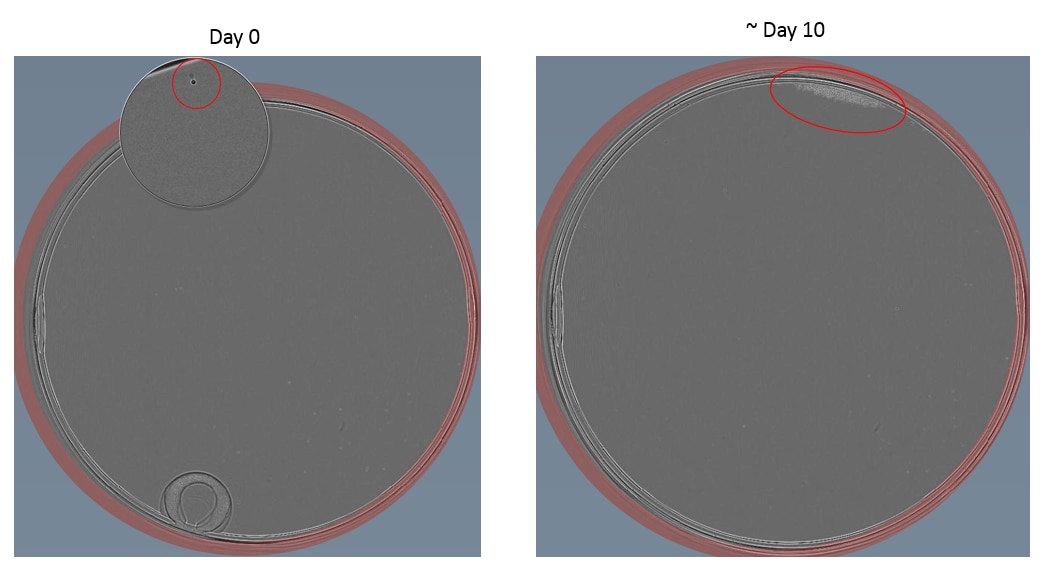
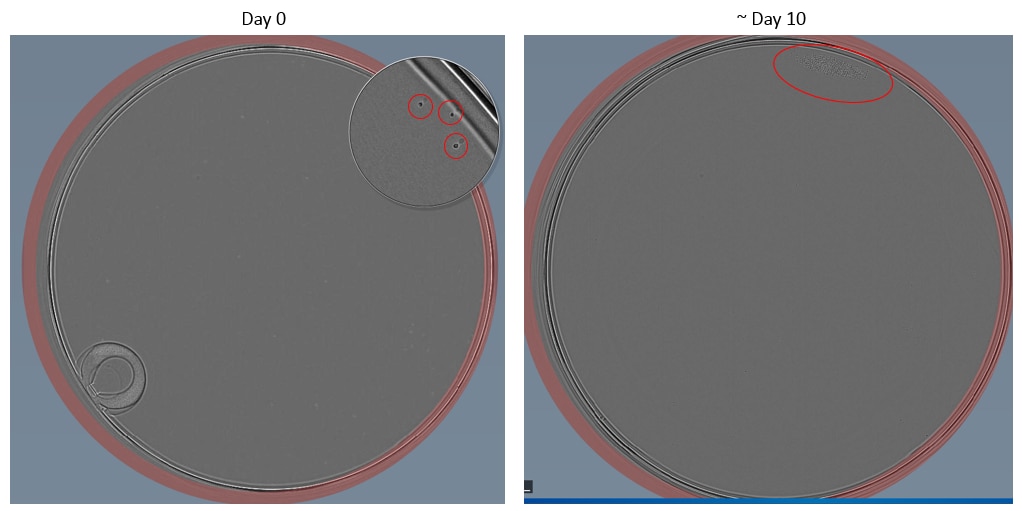
Tips for hard to single cell dilute cell lines
If the cell line does not tolerate single cell dilution in standard culture media, recovery of clones can often be improved by including (up to 50%) conditioned media with the standard culture media.
Conditioned media
Follow the protocol as above but supplement the media with 25-50% conditioned media. Conditioned media should ideally be harvested from healthy cells at no more than 70% confluence at about 48 hours post the previous passage. Conditioned media should be clarified by centrifugation at 1000 x g for 10 minutes in a bench top centrifuge and then passed through a 0.2 μm sterile filter in order to remove any cell debris. Conditioned media can be stored at 4oC for up to 1 week.
Dilution plating
Some adherent cell lines that do not tolerate single cell dilution in 96 well plates can be dilution plated in 10 or 20 cm cell culture dishes. The aim of this is to dilute the cells so that they form single discrete colonies with large gaps between them that can easily be identified by eye and picked. This method allows cells that do not tolerate being on their own in a well to benefit from growth factors and other signals from cells in the dish.
