Successful CRISPR gene editing and siRNA mediated gene silencing require the efficient uptake of the reagent into the cells of interest. Lipid mediated transfection is an easy and rapid way to deliver your reagents into cells. Reverse transfection is a powerful tool for automation and high-throughput screening setups. However, successful experiments require optimized conditions, here we discuss how to set up an experiment to optimize reverse transfection conditions.
There are two main methods of transfection currently used: standard or forward transfection, and reverse transfection. The main difference between techniques is the order of addition of the three necessary components. In a forward transfection cells are seeded on Day 0 and the transfection reagent is added to the cells about 24 h later. In a reverse transfection the transfection reagents are aliquoted and the cells are seeded on top of the reagents on the same day. As reverse transfection reduces hands-on time and can be easily automated this can be especially useful in high-throughput experiments, . Here we will focus on how to optimize a reverse transfection experiment and will demonstrate how to set up a 96 well plate to test 30 different transfection conditions at once.
The first step in any reverse transfection is to set up the reagent plate. For optimization we recommend using two different seeding densities, so we have split the plate in two and duplicate the conditions we have on the right-hand side of the plate with the left.
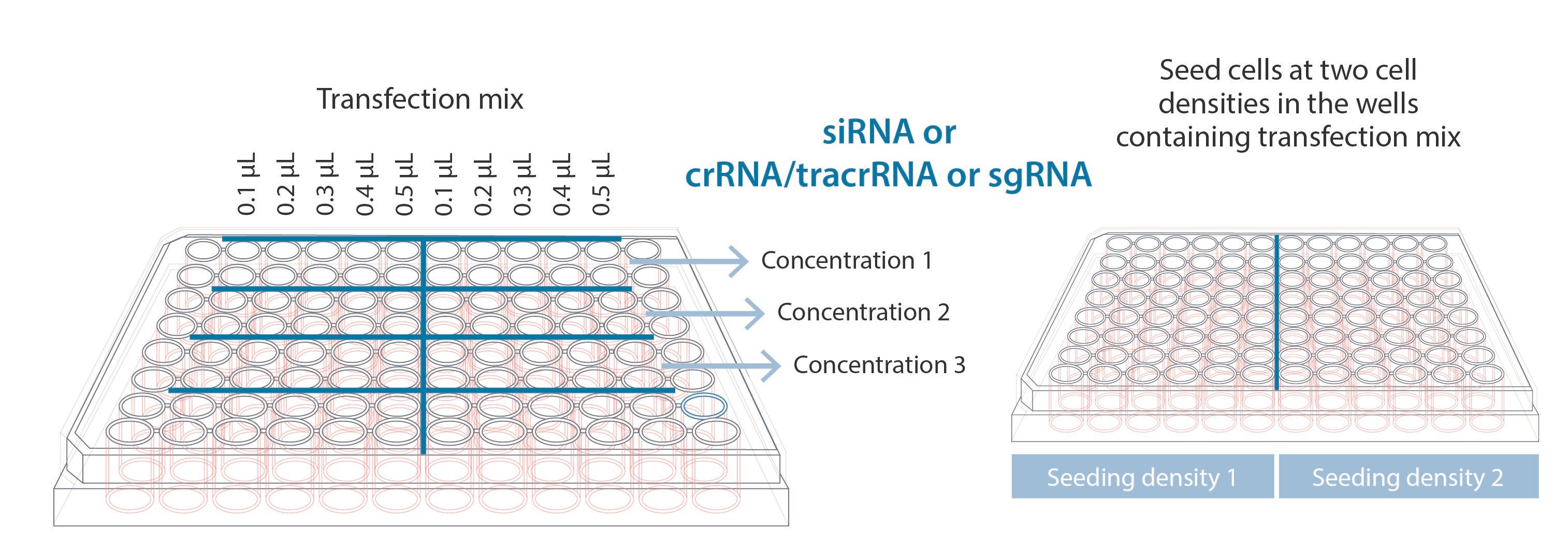
To optimize the reagent input, we recommend testing three different concentrations. This could be of siRNA, crRNA:tracr/Cas9 complex or sgRNA/Cas9 complex depending on the experiment being performed. All our products have recommended working concentrations in their technical manuals. This recommended concentration provides a good midpoint when testing different concentrations.
Finally, we recommend testing 5 different amounts of lipid transfection reagent. In this example we used our DharmaFECT transfection reagent, however a similar strategy could be used with another lipid-based transfection reagent, using the manufacturer’s recommended conditions as a guide to find the middle of the optimization range. After the oligos have complexed with the transfection reagent, seed the two different cell densities into the multi-well plate as shown in the figure. The incubation of the reagents with the cells can vary from 24h – 120h and depends on the type and the doubling speed of the cells. We recommend a robust phenotypic assay such as fluorescence to monitor the success of each condition for quick easy follow up to identify the optimum conditions.
For further information, please refer to our reverse transfection protocol (siRNA / crRNA library). If you need any additional information, please contact our scientific support team who will be glad to help.
 Written by: Ivonne Rubio, Ph.D. | Scientific Support Specialist 3
Written by: Ivonne Rubio, Ph.D. | Scientific Support Specialist 3
Ivonne has been part of the scientific support team since August 2013. With several additional years of hands on experience in the lab and a broad scientific background. She loves assisting customers with their scientific questions, especially about different strategies in gene modulation or CRISPR based studies.
Order Gene editing or siRNA reagents
Browse siRNA offerings
Browse CRISPR offerings
Learn more
- Reverse transfection for high-throughput CRISPR studies Application Page
- Optimization of reverse transfection of Dharmacon™ Edit-R™ synthetic crRNA and tracrRNA components with DharmaFECT™ transfection reagent in a Cas9-expressing cell line – Application note
- Ensure success with appropriate controls in your RNAi experiments – Blog article
