- Gene editing
- Gene editing reagents
- Edit-R Synthetic Positive crRNA Controls
Edit-R synthetic positive crRNA controls
Synthetic crRNA controls to verify optimal parameters for CRISPR-Cas9 gene editing

Please note our synthetic crRNA control catalog numbers changed in 2017 to reflect the addition of stabilizing chemical modifications to the crRNA for nuclease resistance. Learn more about these changes in this featured article.
Edit-R Synthetic crRNA Controls are recommended as positive controls for CRISPR-Cas9 experiments utilizing synthetic crRNA to optimize transfection conditions and verify Cas9 nuclease expression.
Gene-specific positive controls and kits aredesigned and validated for mismatch detection assays to verify gene editing experiments. They configured as follows:
- Individual Edit-R crRNA Control for human or mouse, (5 nmol or 20 nmol) designed against:
- Cyclophilin B (PPIB)
- DNA (cytosine-5)-methyltransferase 3B (DNMT3B)
- Edit-R crRNA Control Kits for human or mouse containing the following:
- Either a 5 nmol or 20 nmol Edit-R CRISPR Synthetic crRNA Control
- 5 nmol Edit-R crRNA Control forward primer
- 5 nmol Edit-R crRNA Control reverse primer
Edit-R Lethal crRNA Controls are universal positive controls designed to induce cell death in a dose- and Cas9-dependent manner by targeting multiple repeat regions in the genome.
Edit-R Lethal control crRNA #1 induces very potent cell death, while Edit-R Lethal control #2 induces moderate cell death. These controls span a large dynamic range and allow for the optimization of CRISPR-Cas9 delivery for observation of both strong and moderate CRISPR-Cas9 phenotypes. Read the Featured Article or our Application Note to learn more about these controls.
Remember to order Edit-R tracrRNA for use with your controls!
Edit-R CRISPR-Cas9 Genome Engineering
The Dharmacon Edit-R platform includes the three critical components required for gene editing in mammalian cells, based on the natural S. pyogenes system:
- A protein, mRNA, or lentiviral vector expressing a mammalian codon-optimized gene sequence encoding Cas9 nuclease
- A chemically synthesized trans-activating CRISPR RNA (tracrRNA), and
- A chemically synthesized CRISPR RNA (crRNA) designed to the gene target site of interest
Once delivered into the cell, the crRNA:tracrRNA complex with Cas9 nuclease to generate site-specific, DNA double-strand breaks (DSBs). When DSBs are repaired through non-homologous end-joining (NHEJ), the resulting small insertions and deletions (indels) can cause nonsense mutations resulting in gene disruption to produce a functional protein knockout.
How much crRNA & tracrRNA do I need?
This table provides the approximate number of experiments that can be carried out for lipid transfection methods at the recommended crRNA:tracrRNA working concentration (25 nM:25nM) in various plate/well formats. Calculations do not account for pipetting errors.| crRNA nmol | tracrRNA nmol | 96-well plate 100 µL reaction volume | 24-well plate 500 µL reaction volume | 12-well plate 1000 µL reaction volume | 6-well plate 2500 µL reaction volume |
|---|---|---|---|---|---|
| 2 | 2 | 800 | 160 | 80 | 32 |
| 5 | 5 | 2000 | 400 | 200 | 80 |
| 10 | 10 | 4000 | 800 | 400 | 160 |
| 20 | 20 | 8000 | 1600 | 800 | 320 |
Loss of cell viability in Cas9-expressing cells treated with Edit-R Lethal crRNA controls
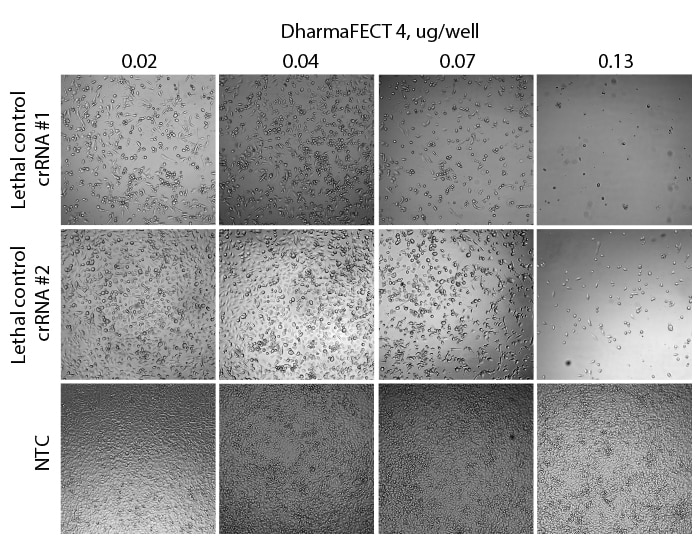
Phase contrast microscopy clearly indicates a strong cell death phenotype with Edit-R crRNA control #1 and a slightly more moderate level of death with Edit-R crRNA control #2 when compared to NTC. The loss in cell viability was dose-dependent on the amount of DharmaFECT 4 transfection reagent used to deliver the crRNA:tracrRNA complex. Cas9-expressing U2OS-Proteasome cells were plated in 96-well plates at 10,000 cells per well. 24 h after plating, cells were transfected with 25 nM crRNA:tracrRNA using 0.02-0.13 µg/well of DharmaFECT 4 transfection reagent. NTC = Non-targeting control.
Optimal transfection conditions for cell death from lethal controls correlate with proteasome-dependent phenotype
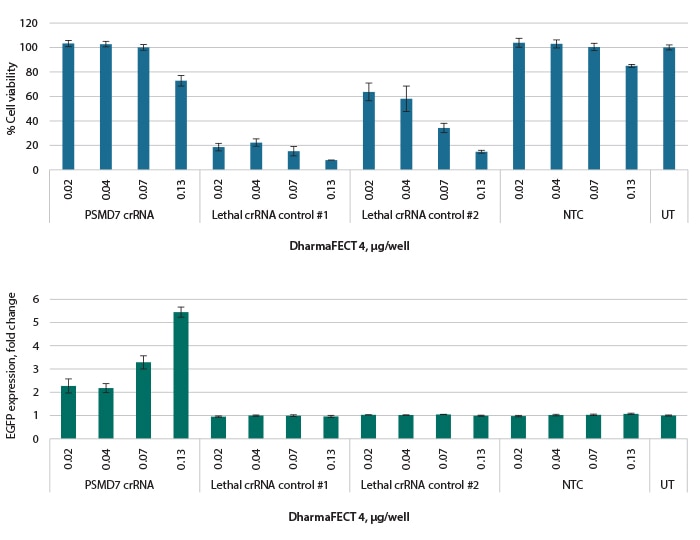
A. Recombinant U2OS Ubi[G76V]-EGFP-Cas9 cells were analyzed for cell viability using a resazurin assay under a range of transfection reagent amounts. Lethal controls show a dose-dependent level of cell death. B. Cells were analyzed for EGFP expression as a readout of proteasome disruption. Optimal conditions indicated by cell viability reproduced optimal EGFP expression. All results were normalized to UT, 72 h after transfection. UT = untreated cells, NTC = Non-targeting control
Effective gene editing of PPIB in human and mouse cells
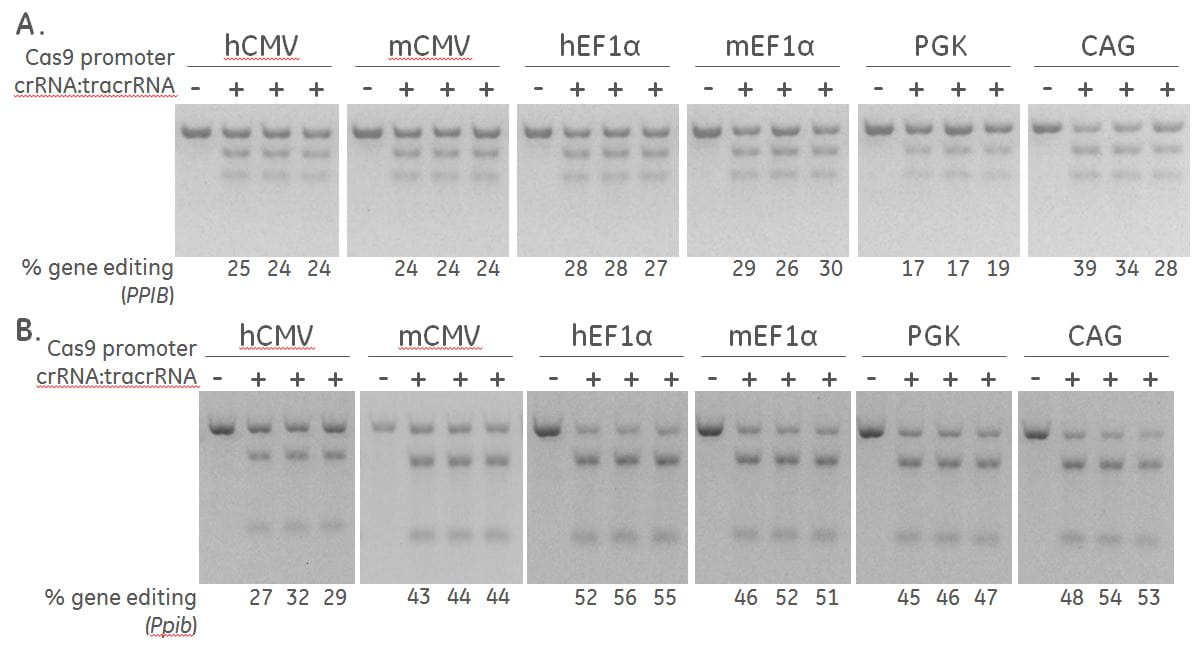
A human recombinant U2OS ubiquitin-EGFP proteasome cell line (Ubi[G76V]-EGFP) (A) and a mouse fibroblast (NIH/3T3) (B), were stably transduced with lentiviral particles containing Cas9 and a blasticidin resistance gene driven by the indicated promoters.. A population of cells with stably integrated Cas9-blastR was selected with blasticidin for a minimum of 10 days before transfections. Cells were transfected with 50 nM synthetic crRNA:tracrRNA targeting Human PPIB / mouse Ppib using DharmaFECT 1 and DharmaFECT 3 Transfection reagent, respectively. After 72 hours, the relative frequency of gene editing was calculated based on a DNA mismatch detection assay using T7EI on genomic DNA extracted from the transfected cells.
Comparable gene editing of PPIB and DNMT3B with either unmodified crRNA:tracrRNA or modified for nuclease resistance
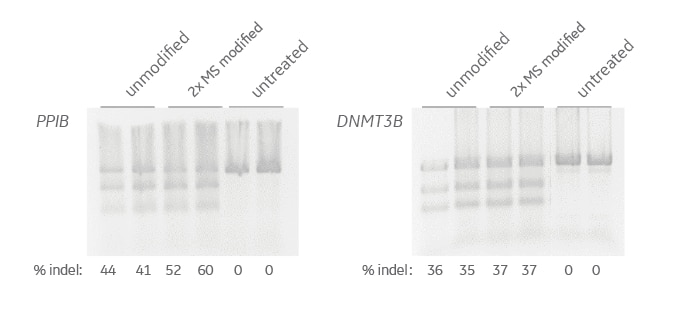
HEK293T cells expressing Cas9 nuclease were transfected with unmodified crRNA:tracrRNA targeting PPIB or DNMT3B or with crRNA:tracrRNA carrying modifications to resist nuclease degradation (2'-O-methyl; 2'OMe) and backbone phosphorothioate linkages (PS) on the two nucleotides at the 5' end of the crRNA and on the two nucleotides at the 3’ end of the tracrRNA). A T7EI DNA mismatch assay was performed and the samples were separated on a 2% agarose gel. Percent indels was calculated and is shown at the bottom of the lanes.
- R. Barrangou, A. Birmingham,et. al.Advances in CRISPR-Cas9 genome engineering: lessons learned from RNA interference.Nucleic Acids Research,43(7) 3407-3419 (2015)
-
M.L. Kelley, Ž. Strezoska, et al. Versatility of chemically synthesized guide RNAs for CRISPR-Cas9 genome editing. J. Biotechnol. 233, 74–83 (2016). doi:10.1016/j.jbiotec.2016.06.011
Application notes
-
Edit-R Lethal crRNA controls for transfection optimization and ongoing monitoring of CRISPR-Cas9 experimental conditions - Application Note
-
Homology-directed repair with Dharmacon Edit-R CRISPR-Cas9 reagents and single-strand DNA oligos - Application Note
-
Optimization of reverse transfection of Edit-R synthetic crRNA and tracrRNA components with DharmaFECT transfection reagent in a Cas9-expressing cell line - Application Note
Posters
-
2'-O-methyl phosphorothioate linkage-modified synthetic guide RNAs for efficient CRISPR-Cas9 genome editing and reduced cellular toxicity - Poster
-
A Synthetic CRISPR-Cas9 System for Homology-Directed Repair - Poster
-
An algorithm for selecting highly functional and specific guide RNAs for CRISPR-Cas9 gene knockout - Poster
-
Complete alignment identification of CRISPR-Cas9 genomic off-targets using Edit-R CRISPR specificity tool - Poster
-
CRISPR-Cas9 genome editing utilizing chemically synthesized RNA - Poster
-
Homology-directed repair with Edit-R CRISPR-Cas9 and single-strand DNA oligos - Poster
Product data
Product inserts
Protocols
Quick protocols
Safety data sheets
Technical manuals
-
CRISPR-Cas9 genome engineering with Cas9 nuclease expression plasmids and Edit-R synthetic RNA - Technical Manual
-
CRISPR-Cas9 genome engineering with inducible lentiviral Cas9 nuclease and Edit-R guide RNAs - Technical Manual
-
CRISPR-Cas9 genome engineering with lentiviral Cas9 particles and Edit-R synthetic guide RNA - Technical Manual
Related Products
Synthetic guide RNAs for efficient gene knockout and unparalleled specificity
Chemically synthesized trans-activating CRISPR RNA required for use with synthetic crRNA for fast and easy gene editing
Cutting control (Safe Harbor) synthetic crRNAs recommended for determination of baseline cellular responses in CRISPR-Cas9 experiments.
Purified Cas9 nuclease mRNA for co-transfection with synthetic guide RNA for a completely DNA-free genome engineering system
