Developing expressing clones with high productivity and stability can be both time-consuming and labor-intensive. The new CHOSOURCE™ TnT transposon technology simplifies cell line development and contributes to the acceleration of biologic development programs.
The CHOSOURCE TnT transposon technology consists of two elements: the TnT transposon vector and the TnT transposase as messenger RNA.
The TnT transposon vector, where the gene of interest (GOI) is cloned and integrates:
- Dual promoters for cloning and expression of multi-chain proteins
- Optimized GS selection cassette to simplify the selection of high expressing clones, with no MSX addition required
- Engineered Terminal Inverted Repeats (TIRs) flanking the expression cassette
CHOSOURCE TnT and CHOSOURCE GS KO and/or ADCC+ cell lines are available in one combined royalty-free license, providing a simple technology access framework without the need for any other third-party technology license.
How does CHOSOURCE TnT transposon technology work?
The TnT transposon vector (containing GOI) and TnT transposase mRNA are co-transfected in the host. After transfection, the TnT transposase recognizes the TIRs and mediates the excision of the gene cassette from the transposon vector (donor) and its integration into transcriptionally active regions of the host genome. This controlled DNA delivery enables highly efficient and stable integration of the GOI into the host genome, increasing cell line performance and clone stability, whilst reducing uncertainty and variability during cell line development.

CHOSOURCE TnT- mechanism of action
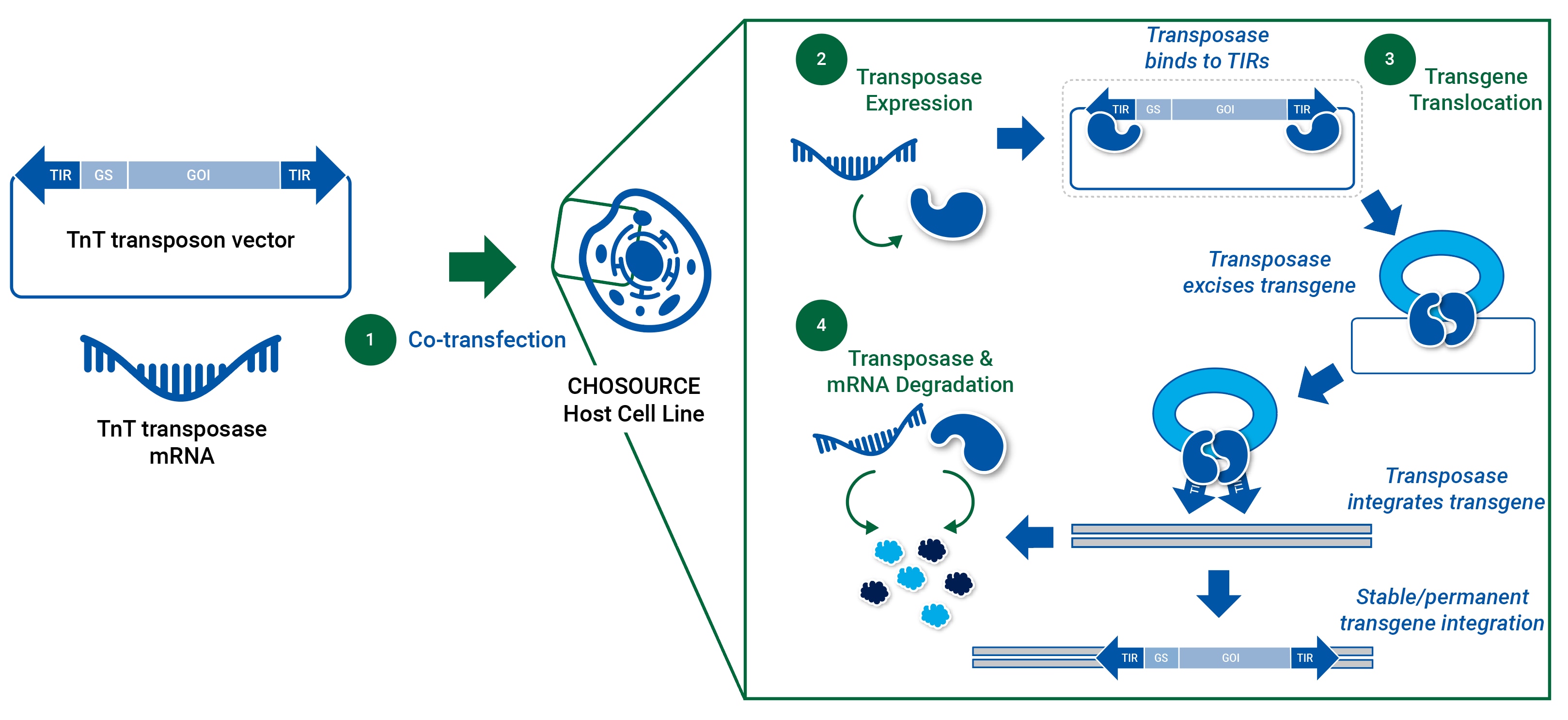
CHOSOURCE TnT- mechanism of action
How can your development programmes benefit from using CHOSOURCE TnT transposon technology?
The CHOSOURCE TnT transposon technology can help you to:
Faster development timelines
Faster recovery time during pool selection and high reproducibility across pools and clones can help to
accelerate cell line development programs.
Safe and reliable genetic modification of host cells
The TnT transposase is delivered as mRNA to eliminate the risk of integration into the host genome.
Transposase enzyme and transposase mRNA are degraded shortly after transfection, avoiding generation of
genomic alterations and/or instabilities.
Minimize risks and delays
The consistent high transfection efficiency and clonal stability achieved with
CHOSOURCE TnT virtually eliminates unexpected setbacks during cell line and process
development.
Streamline process development
CHOSOURCE TnT allows users to effortlessly generate stable expressing clones without the use of MSX or any other selection agent. The high reproducibility observed at pool and clone levels, reduces the efforts required for screening many pools and/or clones, thus simplifying CLD process.
Disruptive development and manufacturing workflows
The robustness and reliability of CHOSOURCE TnT makes it a great tool to streamline development
and manufacturing efforts, potentially allowing new development paradigms to expedite preclinical
(and potentially clinical) development.
Simplify your cell line development
Contact us
Ready to discuss your mission critical project with us?
Download the Application Note
Streamlining the development of bispecific antibodies from expression to quality assessment with Revvity’s biotherapeutic workflow solutions
See the data showing how the CHOSOURCE TnT Transposon Technology was used to express an asymmetric 4-chain bispecific antibody in the CHOSOURCE GS KO host cell line
Download the Scientific Poster
Integration of next-generation transposon vectors with novel host cell lines
The data presented shows the performances of CHOSOURCE TnT with CHOSOURCE CHO-K1 GS KO and ADCC+ cell lines, when expressing a standard immunoglobulin G (IgG) molecule.
Download the White Paper
Leveraging next-generation cell line development technologies for cost-effective biotherapeutic applications
Read how cell line development challenges can be addressed with advanced technologies covering how to prevent clone instability, achieve acceptable protein expression, and allow for non-fragmented, multi-copy gene cassette integration.

