
WHEREVER YOU STAND ON THE INTERSECTION OF SCALE AND COMPLEXITY, we can help you move your research forward quickly. Cell line engineering has opened a new world of possibilities, Horizon Discovery offers cell variations at automated scale and complex engineering from seasoned experts, so you can stay focused on inventing the future.
Partner with us for nearly any edit, in any cell line.
When it’s time to take your research to the next level, Horizon Discovery is there for you.
Technical expertise to tackle complexity
- Over 10 years of experience
- 1000’s of edits across 100’s of cell types
- Scientific consultation to start solving problems before they arise
Flexible & Responsive to your needs
- Simple to complex edits
- Scalable through automation
- Choose your cell line: 100’s pre-licensed cell lines available (see licensing tab below) or edit your own
- Pools or clones (project dependent)
Peace of mind so you can focus on the biology
- Comprehensive data package
- Sequence verification
- Mycoplasma & Sterility testing

Project options
- Single knockout
- Double knockout
- Small defined deletion
- Large genomic deletion
- Point mutation (SNP) knock-in
- Defined zygosity available (additional cost)
- Tag / Reporter knock-in
- Turbo GFP (Evrogen), Turbo RFP (Evrogen), or other fluorescent reporters
- His, Myc, or FLAG
- HiBiT (Promega), LgBiT (Promega)
- NanoLUC®: Protein Reporter (Promega), or NanoLUC®: Promoter Reporter (Promega)
- Defined N- or C-terminal insert location
- Large knock-in
- iPS cell editing
- Single knockout
- Point mutation (SNP) knock-in
- Tag / Reporter knock-in
- Large knock-in
- Difficult to edit cell lines
- Random integration – Cas9, overexpression and more
- CRISPR-mediated genomic translocations
- Complex modifications – large knock-ins, large deletions and more
- Don't see an option that meets your needs? Contact us and we will be in touch to discuss further – we can perform nearly any edit, in any cell line.
Manufacturing Workflow
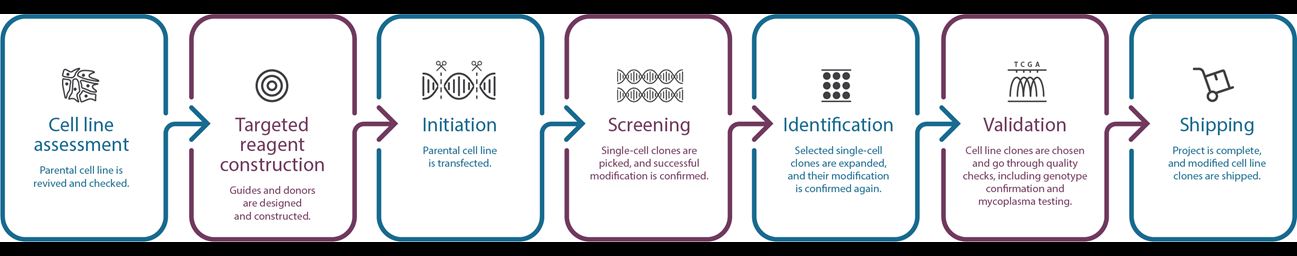
- Customer can choose from one of our in-house cell lines, or provide a cell line to onboard. During the onboarding process, we perform mycoplasma and sterility testing, as well as assess transfectability and single cell dilution growth characteristics.
- In parallel with the cell line assessment phase, we also design and evaluate reagents to be used in the manufacturing process.
- During the cell line engineering phase, our team performs the desired edit(s) defined in the statement of work. Throughout the process, our Project Management team provides regular updates and will contact you immediately if any issue or complication should arise during the manufacturing process.
- After manufacturing is complete, the cell line is validated for the desired edit. As standard, we validate at the genomic level with Sanger sequencing and for our reporter cell lines we provide a functional readout that verifies reporter expression.


