Immune cell screening services
Answer questions in biologically relevant primary immune cell types. Leveraging our expertise in screening and receive robust data from high-throughput assays.
| Service description | Number of suitable compounds | Catalog Number |
|---|---|---|
| Standard iTreg Polarization Assay - Mini | 1 to 5 | IMM04-01 |
| Standard iTreg Polarization Assay - Midi | 6 to 11 | IMM04-02 |
| Standard iTreg Polarization Assay - Maxi | 12 to 17 | IMM04-03 |
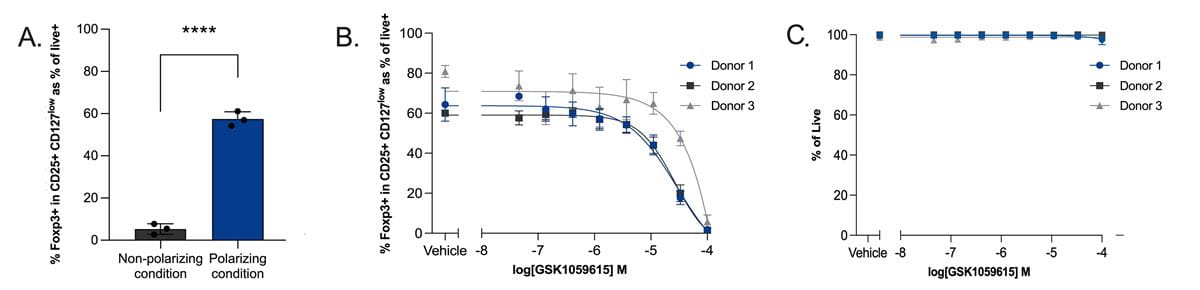
A) Optimization of naïve CD4+ T cell polarizing condition in the 384-well plate format. Data represent quadruplicate technical repeats cultured from three donors. B) Drug-modulated iTreg polarization by GSK1059615 during polarization reduced the expression of Foxp3. Flow cytometry data represent naïve T cells cultured from 3 donors in quadruplicate replicates. C) Viability assessment of cells treated with GSK1059615 in the iTreg polarization assay.