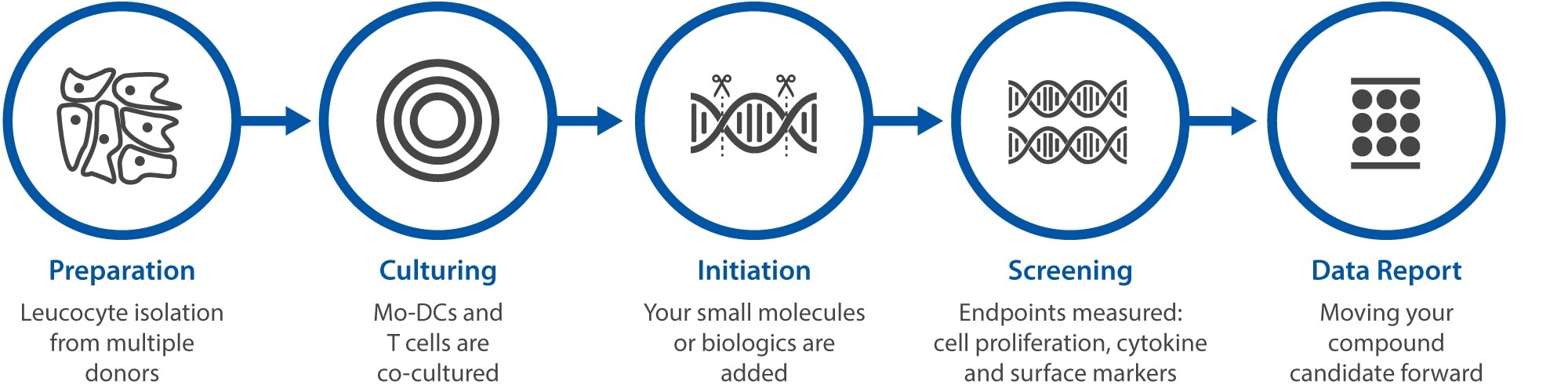
Horizon’s Standard MLR assay services
Send us your biologics or small molecules, and our proven method will analyze its effects on multiple endpoints from multiple allogeneic donors. We designed optimised 3-fold dose response of 8 points testing up to 16 compounds from a single set of primary cells.
- Short project completion – from 4 weeks
- Donor-donor reproducibility
- Robust data pack
Standard MLR assay read-outs
The data you will get include:
- Interferon-gamma (IFN-γ) cytokine release
- T cell viability
- T cell proliferation by flow cytometry of:
- % CTV of CD4+
- % CTV of CD8+
- % CD25
Our cloud-based data report include
- Cell quality control metrics
- Assay performance QC
- Response curves plots
- Raw Data and Metadata
| Service description |
Number of suitable compounds |
Catalog Number |
| Standard MLR assay - Mini |
1 to 4 |
IMM01-01 |
| Standard MLR assay - Midi |
5 to 10 |
IMM01-02 |
| Standard MLR assay - Maxi |
11 to 16 |
IMM01-03 |
Request pricing for the three sizes available and a sales representative will be in touch within 48 hours.


