For early drug discovery target identification working with primary immune cells, leverage an industry-leading reproducible and functional relevant T cell arrayed screen that will advance your therapeutic development.
CRISPR knock-out gene screening offers the potential for revealing therapeutic targets and understanding genetic disease links. With a T cell arrayed screen you can thoroughly understand the impact of gene suppression on cell function within in vitro settings and overcome the challenge seen when manipulating primary immune cells with complex gene interplays vital for immune responses.
What is an arrayed T cell CRISPR KO screen?
It is a specialized genetic screening approach that employs CRISPR-Cas9 gene-editing technology to systematically disrupt or "knock out" specific genes within T cells. In an arrayed screening setup, each sample contains a single KO gene, enabling in-depth analysis of that specific gene with multiplexed readouts, including proliferation (CTG), surface and intracellular markers (FACS), and protein release quantification (HTRF®). The automation and shorter timelines provided by the high-throughput platform allow for the use of cells with a single-gene knockout in downstream functional assays.
CRISPR KO applications in T lymphocytes
- Investigate gene roles in a CRISPR-modulated immune set-up.
- Assess compound mode of action in a CRISPR-modulated immune in vitro model.
- Uncover gene-phenotype/cell interactions and study their effects on T cell behavior.
Our T cell CRISPR KO screening platform
We go beyond traditional approaches by combining the power of CRISPR knockout technology with the reliability of T cell ImmuSignature™ assays. This distinctive service combination unleashes the full potential of our T cell gene screening, offering robust statistical analysis alongside relevant in vitro immune cell functional assessments to enhance your target discovery efforts.
- Advanced miniaturized pipeline enabling the assessment of drug-phenotype interactions.
- Designed for the screening of bi-specifics, small molecules, or monoclonal antibodies.
- Expandable to additional functional read-outs, such as cytokine release through HTRF® technology.
CRISPR T cell arrayed screening workflow
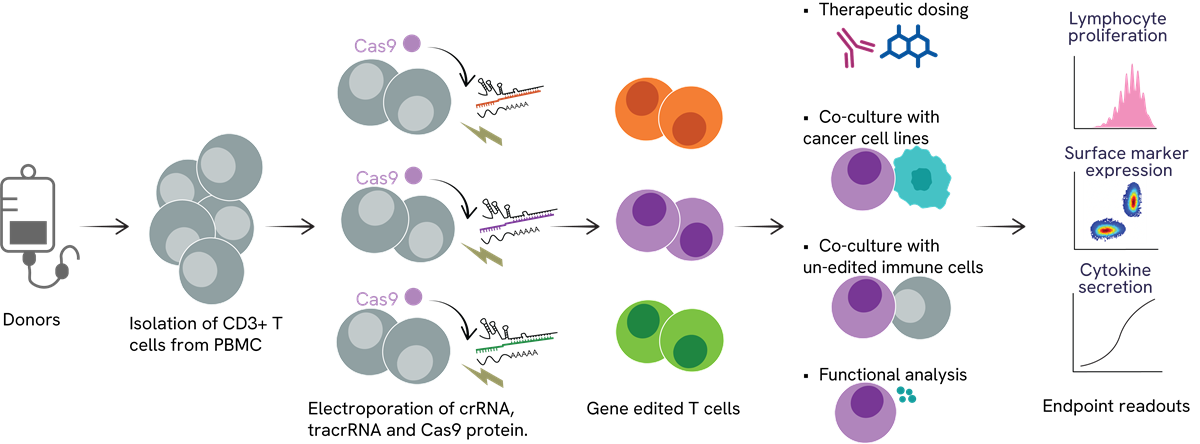
Description of a semi-automated T cell gene editing platform with functional assessments. This platform facilitates the discovery of novel gene targets in immune responses, aiding the targeting of critical genes in immune-related disorders. In the arrayed screen, stimulated T cells undergo electroporation with crRNA, tracrRNA, and Cas9 protein. Edited T cells can then be co-cultured with unedited immune cells and cancer cell lines or subjected to a therapeutic candidate. Analysis in each well includes measuring proliferation, surface marker expression, and cytokine release.
Functional analysis by T cell activation assay of edited cells
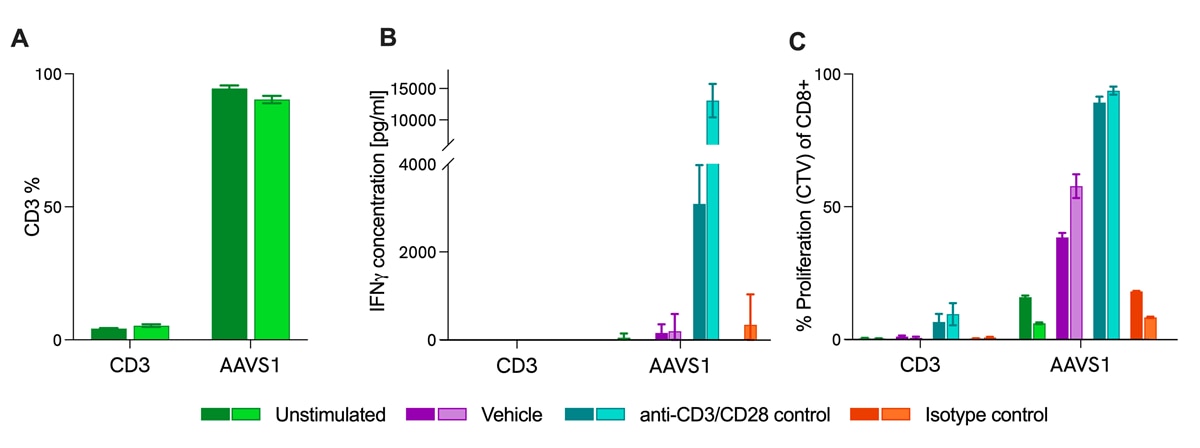
CD3 KO T cells show cytokine release and proliferation defects in a CRISPR-ImmuSignature™ T cell activation (TCA) project. Human CD3 T cells were targeted with CD3 CRISPR guide RNA and Cas9 protein in an arrayed screening format. Edited cells were stimulated with anti-CD3 and anti-CD28 antibodies in the ImmuSignatureTM T cell activation (TCA) assay. A) Knock-out efficiency of CD3 measured by flow cytometry surface expression percentage of total lymphocytes. B) Impaired INF-y secretion of edited CD3 KO measured by HTRF® technology. C) Reduced proliferation of CD3 KO CD8 T cell populations assessed by CTV in flow cytometry. AAVS1: safe harbor control. Two donors are depicted in all conditions.

