Guidelines for selection of sgRNAs for protein knockout experiments based on nCas9 (NGG PAM requirements) and rat APOBEC deaminase.
-
Using manual methods or publicly available base editing guide RNA design tools (see example resources section, or appendix A), generate lists of candidate sgRNA protospacer (targeting) sequences for your purpose based on the transcript of interest:
- Generate premature stop codons for protein knockout by designing sgRNAs with NGG PAMs that allow the conversion of CAA, CAG, CGA, or TGG codons into STOP codons (Figure 1) when the target C(s) are located within position 3 to 8 of the spacer sequence in the 5'-3' direction (Figure 2)1.
- Generate splice site disruptions for protein knockout:
- Design sgRNAs with an NGG PAM that locate the target C within position 3 to 8 of the spacer sequence in the 5'-3' direction (Figure 2)1;
- Splice acceptor site is generally: intron-AG|exon;
- Splice donor site is generally exon|GT-intron;
- Converting the GC base pair to an AT base pair will lead to splice site disruption2.
-
Further refine candidate lists by the following criteria, if necessary:
- Consider your gene structure to select a subset of gRNAs with highest probability to result in protein knockout:
- Avoid gRNAs that target the earliest and/or latest exons;
- Give higher priority to gRNAs that targets the middle part of the protein, with preference for gRNAs located within the middle 10-60% of your transcript – if necessary to find additional gRNA candidates, look beyond 60% into the CDS until sufficient guides are found;
- Give preference to gRNAs that target exon coding for essential domains in the protein (i.e transmembrane domain for receptors);
- Target non-symmetric exons. If a symmetric exon is targeted, and the targeted exon is skipped, the following exons will be in-frame3;
- Ensure the sgRNA targets all required isoforms.
- Prioritize guides based on the 2 nt 5' of the target C3:
- Give preference where the sequence context is NTC and TCC;
- Avoid where the sequence context is NGC and GCC.
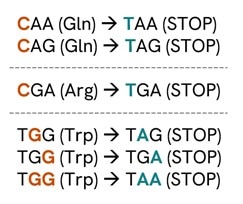
Figure 1. Stop codons that can be generated from a cytidine deamination1

Figure 2. Base editing window. Dark green= potential for highest efficiency editing. Light green= potential for lower efficiency editing. White= potential for bystander editing.
References
-
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5610906 (see figure 1)
-
https://www.nature.com/articles/s41467-021-22009-2 (see figure 1)
-
Poster - Performance and modularity of Horizon’s Pin-point™ base editing system characterized by arrayed and pooled screening platforms
Additional External Resources
-
BE-Designer http://www.rgenome.net/be-designer/
-
iSTOP https://www.ciccialab-database.com/istop/#/
-
PnB Designer https://fgcz-shiny.uzh.ch/PnBDesigner/
-
beditor https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6553823/ (requires the use of Python)
-
SpliceR https://pubmed.ncbi.nlm.nih.gov/33893286/
-
CRISPR-BETS https://zhangtaolab.org/software/crisprbets
All custom sgRNA sequences must be experimentally validated. These guidelines and suggestions are without guarantee of functionality. Specificity of sgRNA sequences should always be considered to reduce off-target effects.
Revvity provides, and recipient accepts, this information “as is”. Recipient accepts all responsibility for the use of the information including responsibility for determining whether such use may require third party intellectual property rights.
This document provides links or otherwise provide access to another website, mobile application, or Internet location (collectively "Third-Party Sites"). We provide these links merely for your convenience. We have no control over, do not review, and are not responsible for Third-Party Sites, their content, or any goods or services available through the Third-Party Sites. Our Privacy Notice does not apply to Third-Party Sites, and any data you provide to Third-Party Sites, you provide at your own risk. We encourage you to review the privacy policies of any Third-Party Sites with which you choose to interact.
Order custom sgRNAs
Download this guideline as a PDF
Designing custom single guide RNAs (sgRNAs)
Guidelines for selection of sgRNAs for protein knockout experiments based on nCas9 (NGG PAM requirements) and rat APOBEC deaminase.
Need more help?
Contact UsThe rat APOBEC deaminase can either be used to convert a C to T or a G to A. Examples of both are provided below.
Example 1: Targeting a C
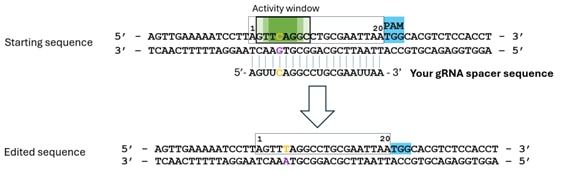
- Identify the nucleotide to edit (in orange); in this example CAG is converted to a STOP codon (TAG). Consider that the NGG PAM is always located at the 3' of the spacer on the targeted strand*.
- Ensure it is positioned in the activity window (darker green = potential for highest efficiency editing).
- Your spacer sequence will bind the non-targeted sequence and should be complementary to it.
- Note that sequences are input form 5' to 3' and as DNA sequences (T instead of U).
In this example your input sequence would be: 5'- AGTTCAGGCCTGCGAATTAA - 3'
Example 1: Targeting a G
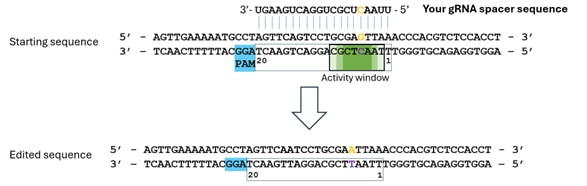
- Identify the nucleotide to edit (in orange); in this example a splice acceptor site (AG) is mutated. Consider that the NGG PAM is always located at the 3' of the spacer on the targeted strand*.
- Ensure it is positioned in the activity window (darker green = potential for highest efficiency editing).
- Your spacer sequence will bind the non-targeted sequence and should be complementary to it.
- Note that sequences are input form 5' to 3' and as DNA sequences (T instead of U).
In this example your input sequence would be: 5'-TTAACTCGCTGGACTGAAGT-3'
*targeted stand = the strand where the C substrate of the deaminase is located
