Screening therapeutic antibody candidates for complement-dependent activity can be crucial in developing successful biologicals. Take advantage of Horizon´s extensive cell line library and challenge your compounds in a semi-automated manner and progress with confidence
What is a Complement-Dependent Cytoxicity (CDC) assay?
Complement-dependent cytotoxicity (CDC) is an immune response in which target cells are lysed through activation and recruitment of the complement cascade to the targeted cell surface. The CDC is a tightly regulated process, where several therapeutic monoclonal antibodies leverage the power of the membrane attack complex to lyse cancerous cells.
There are many reasons why the CDC assay could be a good option for your project
The CDC assay supports the validation of therapeutic antibodies for cancer immunotherapy to confirm complement-dependent effectiveness. The lead therapeutics compounds can be screened in model cancer cell lines to identify the mechanism of action. Moreover, screening of therapeutic candidates in a broader OncoSignature™ panel of cancer cell lines can expand the applications of your lead compound in other cell types or tissues.
Horizon's CDC assay
Our CDC assay allows screening of a panel of compounds in a model cancer cell line to identify candidate lead compounds for more advanced studies. Alternatively, screen a few lead compounds in a cancer cell line panel to test lead compounds' effectiveness in different cell types/tissues.
Key highlights:
- Rapid and robust data of your compounds’ cytotoxic activity from an in vitro setting
- Short project completion – from 5-6 weeks (dependent on cell growth)
- Screen panel of candidate therapeutics in model cell lines
- Validate lead therapeutics in OncoSignature™ cancer cell line panel
Request a quote
Discuss your project with our team
From planning and execution to data analysis, we have the expertise to move your project forward.
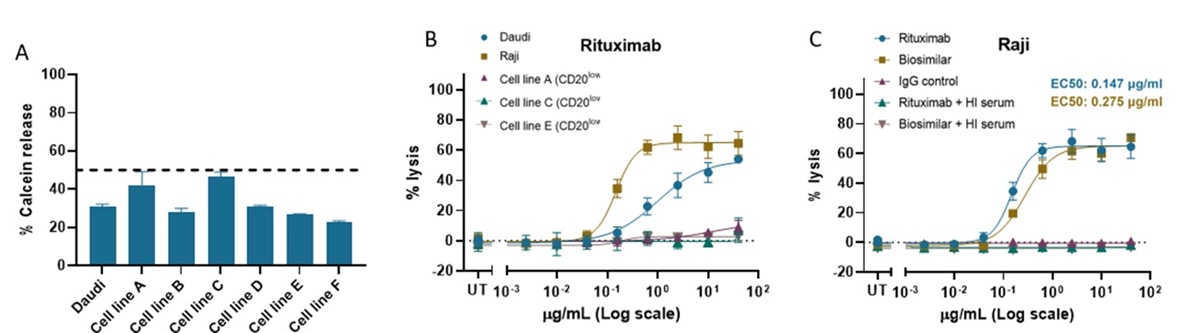
Supporting data
Download app note
Learn more about the CDC assay and analysis data.
Immunology services
Browse immunology assays
Get reliable data from primary immune cells
Read Horizon's Immunology blog
Read Horizon's Immunology blog
Get the latest insights about the drug discovery tools in the immunology field curated by Horizon’s scientists
The CDC assay is a powerful screening platform to aid the development of successful therapeutic mAbs. Here, we have developed a robust CDC assay workflow incorporating semi-automation, which accurately and reproducibly supports the testing and comparison of mAbs candidates across a panel of cancer cell lines. Leveraging OncoSignature™ cell line panel, we can apply our CDC assay workflow to test mAbs targeting a broad range of surface proteins with various expression levels, thus ensuring that candidate mAbs can be validated across several cell lines and lineages.